Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Palaeoscolecida and the Evolution of the Ecdysozoa Andrey Yu
The Palaeoscolecida and the evolution of the Ecdysozoa Andrey Yu. Zhuravlev1, José Antonio Gámez Vintaned2 and Eladio Liñán1 1Área y Museo de Paleontología, Departamento de Ciencias de la Tierra, Facultad de Ciencias, Universidad de Zaragoza, C/ Pedro Cerbuna, 12, E-50009 Zaragoza, Spain 2Área de Paleontología, Departamento de Geologica, Facultad de Ciencias Biológicas, Univeristat de València, C/ Doctor Moliner, 50, E-46100 Burjassot, Spain Email: [email protected] AbstrAct rÉsUMÉ Palaeoscolecidans are a key group for understanding the ear- Les Paléoscolécides sont un groupe clé pour la compréhen- ly evolution of the Ecdysozoa. The Palaeoscolecida possess sion des débuts de l’évolution des Ecdysozoa. Les Pal- a terminal mouth and an anus, an invertible proboscis with aeoscolecida possèdent une bouche terminale et un anus, pointed scalids, a thick integument of diverse plates, sensory un proboscis inversible aux scalides pointues, un tégument papillae and caudal hooks. These are features that draw a épais de plaques diverses, des papilles sensorielles et des secret out of these worms, indicating palaeoscolecidan af- crochets caudaux. Ceux-ci sont des traits qui tirent un secret finities with the phylum Cephalorhyncha, which embraces de ces vers, ce qui indique des affinités paléoscolecides avec priapulids, kinorhynchs, loriciferans and nematomorphs. At le phylum des Cephalorhyncha qui inclut les priapulides, les the same time, the Palaeoscolecida share a number of char- kinorhynches, les loricifères et les nématomorphes. Cepen- acters with the lobopod-bearing Cambrian ecdysozoans, the dant, les Palaeoscolecida ont aussi quelques-uns des mêmes Xenusia. Xenusians commonly possess a terminal mouth, caractères que les Xenusia, ces écdysozaires cambriens qui a proboscis (although not retractable), and a thick integu- portaient des lobopodes. -

An Ordovician Lobopodian from the Soom Shale Lagerstätte, South Africa
[Palaeontology, Vol. 52, Part 3, 2009, pp. 561–567] AN ORDOVICIAN LOBOPODIAN FROM THE SOOM SHALE LAGERSTA¨ TTE, SOUTH AFRICA by ROWAN J. WHITTLE*,à, SARAH E. GABBOTT*, RICHARD J. ALDRIDGE* and JOHANNES THERON *Department of Geology, University of Leicester, Leicester LE1 7RH, UK; e-mails: [email protected]; [email protected] Department of Geology, University of Stellenbosch, Private Bag XI, Stellenbosch 7602, South Africa; e-mail: [email protected] àPresent address: British Antarctic Survey, Madingley Road, Cambridge, CB3 0ET, UK; e-mail: [email protected] Typescript received 18 July 2008; accepted in revised form 27 January 2009 Abstract: The first lobopodian known from the Ordovician and Carboniferous. The new fossil preserves an annulated is described from the Soom Shale Lagersta¨tte, South Africa. trunk, lobopods with clear annulations, and curved claws. It The organism shows features homologous to Palaeozoic mar- represents a rare record of a benthic organism from the ine lobopodians described from the Middle Cambrian Bur- Soom Shale, and demonstrates intermittent water oxygena- gess Shale, the Lower Cambrian Chengjiang biota, the Lower tion during the deposition of the unit. Cambrian Sirius Passet Lagersta¨tte and the Lower Cambrian of the Baltic. The discovery provides a link between marine Key words: Lagersta¨tte, lobopodian, Ordovician, Soom Cambrian lobopodians and younger forms from the Silurian Shale. Cambrian lobopodians are a diverse group showing a in an intracratonic basin with water depths of approxi- great variety of body shape, size and ornamentation. mately 100 m (Gabbott 1999). Dominantly quiet water However, they share a segmented onychophoran-like conditions are indicated by a lack of flow-induced sedi- body, paired soft-skinned annulated lobopods, and in mentary structures and the taphonomy of the fossils. -

Lobopodian Phylogeny Reanalysed
BRIEF COMMUNICATIONS ARISING Phylogenetic position of Diania challenged ARISING FROM J. Liu et al. Nature 470, 526–530 (2011) Liu et al.1 describe a new and remarkable fossil, Diania cactiformis. absent. For example, character 6 (position of frontal appendage) can This animal apparently combined the soft trunk of lobopodians (a only be coded in taxa that possess a frontal appendage (character 5) in group including the extant velvet worms in addition to many the first instance (such that a ‘‘0’’ for character 5 necessitates a ‘‘-’’ for Palaeozoic genera) with the jointed limbs that typify arthropods. character 6). In morphological analyses such as this, inapplicable They go on to promote Diania as the immediate sister group to the states are usually assumed to have no bearing on the analysis, being arthropods, and conjecture that sclerotized and jointed limbs may reconstructed passively in the light of known states. In analyses of therefore have evolved before articulated trunk tergites in the imme- nucleotide data, by contrast, gaps may alternatively be construed as a diate arthropod stem. The data published by Liu et al.1 do not un- fifth and novel state, because shared deletions from some ancestral ambiguously support these conclusions; rather, we believe that Diania sequence may actually be informative. If this assumption is made with probably belongs within an unresolved clade or paraphyletic grade of morphological data, however, all the logically uncodable states in a lobopodians. character are initially assumed to be homologous, and a legitimate Without taking issue with the interpretation of Diania offered by basis for recognizing clades. -

Hallucigenia's Onychophoran-Like Claws
LETTER doi:10.1038/nature13576 Hallucigenia’s onychophoran-like claws and the case for Tactopoda Martin R. Smith1 & Javier Ortega-Herna´ndez1 The Palaeozoic form-taxon Lobopodia encompasses a diverse range of Onychophorans lack armature sclerites, but possess two types of ap- soft-bodied‘leggedworms’ known from exceptionalfossil deposits1–9. pendicular sclerite: paired terminal claws in the walking legs, and den- Although lobopodians occupy a deep phylogenetic position within ticulate jaws within the mouth cavity9,23.AsinH. sparsa, claws in E. Panarthropoda, a shortage of derived characters obscures their evo- kanangrensis exhibit a broad base that narrows to a smooth conical point lutionary relationships with extant phyla (Onychophora, Tardigrada (Fig. 1e–h). Each terminal clawsubtends anangle of130u and comprises and Euarthropoda)2,3,5,10–15. Here we describe a complex feature in two to three constituent elements (Fig. 1e–h). Each smaller element pre- the terminal claws of the mid-Cambrian lobopodian Hallucigenia cisely fills the basal fossa of its container, from which it can be extracted sparsa—their construction from a stack of constituent elements— with careful manipulation (Fig. 1e, g, h and Extended Data Fig. 3a–g). and demonstrate that equivalent elements make up the jaws and claws Each constituent element has a similar morphology and surface orna- of extant Onychophora. A cladistic analysis, informed by develop- ment (Extended Data Fig. 3a–d), even in an abnormal claw where mental data on panarthropod head segmentation, indicates that the element tips are flat instead of pointed (Extended Data Fig. 3h). The stacked sclerite components in these two taxa are homologous— proximal bases of the innermost constituent elements are associated with resolving hallucigeniid lobopodians as stem-group onychophorans. -

Hallucigenia's Head and the Pharyngeal Armature Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Apollo 1 Hallucigenia’s head and the pharyngeal armature of early ecdysozoans 2 Martin R. Smith1 and Jean-Bernard Caron2,3 3 1Department of Earth Sciences, University of Cambridge, Cambridge, CB2 3EQ, UK. 4 <[email protected]> 5 2Department of Natural History (Palaeobiology Section), Royal Ontario Museum, Toronto, 6 ON M5S 2C6, Canada. <[email protected]> 7 3Department of Ecology and Evolutionary Biology and Earth Sciences, University of 8 Toronto, Toronto, ON M5S 3B2, Canada. 9 Keywords: Hallucigenia, Ecdysozoa, Panarthropoda, Onychophora, Lobopodia, Burgess 10 Shale, Cambrian explosion. 11 The molecularly-defined clade Ecdysozoa1 comprises the panarthropods 12 (Euarthropoda, Onychophora, and Tardigrada) and the cycloneuralian worms 13 (Nematoda, Nematomorpha, Priapulida, Loricifera, and Kinorhyncha). These 14 disparate phyla are united by their means of moulting, but otherwise share few 15 morphological characters – none of which has a meaningful fossilization potential. As 16 such, the early evolutionary history of the group as a whole is largely uncharted. Here 17 we redescribe the 508 million year old stem-group onychophoran Hallucigenia sparsa2–6 18 from the mid-Cambrian Burgess Shale. We document an elongate head with a pair of 19 simple eyes, a terminal buccal chamber containing a radial array of sclerotized 20 elements, and a differentiated foregut that is lined with acicular teeth. The radial 21 elements and pharyngeal teeth resemble the sclerotized circumoral elements and 22 pharyngeal teeth expressed in tardigrades7–9, stem-group euarthropods10–12, and 23 cycloneuralian worms13. Phylogenetic results indicate that equivalent structures 24 characterized the ancestral panarthropod and, seemingly, the ancestral ecdysozoan – 25 demonstrating the deep homology of panarthropod and cycloneuralian mouthparts, and 26 providing an anatomical synapomorphy for the ecdysozoan supergroup. -

The Xenusian-To-Anomalocaridid Transition Within the Lobopodians
Bollettino della Società Paleontologica Italiana, 50 (1), 2011, 65-74. Modena, 1 luglio 201165 The xenusian-to-anomalocaridid transition within the lobopodians Jerzy DZIK J. Dzik, Instytut Paleobiologii PAN, Twarda 51/55, 00-818 Warszawa, and Instytut Zoologii Uniwersytetu Warszawskiego, Banacha 2, 02-079 Warszawa, Poland; [email protected] KEY WORDS - Lobopods, Arthropods, Origin, Evolution, Cambrian. ABSTRACT - The morphological series composed of large xenusiids of the Chengjiang fauna of China and the basal anomalocaridids Pambdelurion and Kerygmachela from the Sirius Passet fauna of Greenland is supplemented with another xenusiid lobopodian, Siberion lenaicus gen. et sp. nov., from the Early Cambrian Sinsk Formation of central Siberia. Reduction and ventral bending of the proboscis in Siberion and the Chengjiang Megadictyon and Jianshanopodia may be a synapomorphy uniting these representatives with the anomalocaridids. Throughout the series, the raptorial appendages became larger and more sclerotised, while the gill-like structures on the trunk appendages were transformed from their originally tubular shape into a pinnate form and may eventually have given rise to the wide anomalocaridid flaps. Such a tendency can be rooted in the Aysheaia-like xenusians, that have raptorial appendages associated with a prominent proboscis. This results in a scenario of almost complete transition from early lobopodians to ancestral arthropods within the xenusian-anomalocaridid segment of the phylogenetic tree. RIASSUNTO - [Il passaggio evolutivo da xenusiidi ad anomalocarididi all’interno dei lobopodi] - La sequenza morfologica costituita dai grandi xenusiidi presenti nella fauna cinese di Chengjiang e dai primi anomalocarididi appartenenti ai generi Pambdelurion e Kerygmachela presenti nella fauna del Sirius Passet della Groenlandia viene integrata da un altro lobopode xenusiide, Siberion lenaicus gen. -

Phylogenetic Position of Diania Challenged
Mounce, R. C. P. and Wills, M. A. (2011) Phylogenetic position of Diania challenged. Nature, 476 (7359). E1-E4. ISSN 0028-0836 Link to official URL (if available): http://dx.doi.org/10.1038/nature10266 Opus: University of Bath Online Publication Store http://opus.bath.ac.uk/ This version is made available in accordance with publisher policies. Please cite only the published version using the reference above. See http://opus.bath.ac.uk/ for usage policies. Please scroll down to view the document. BRIEF COMMUNICATIONS ARISING ; Phylogenetic position of Diania challenged ARISING FROM J. Liu et al. Nature 470, 526–530 (2011) Liu et al.1 describe a new and remarkable fossil, Diania cactiformis. absent. For example, character 6 (position of frontal appendage) can This animal apparently combined the soft trunk of lobopodians (a only be coded in taxa that possess a frontal appendage (character 5) in group including the extant velvet worms in addition to many the first instance (such that a ‘‘0’’ for character 5 necessitates a ‘‘-’’ for Palaeozoic genera) with the jointed limbs that typify arthropods. character 6). In morphological analyses such as this, inapplicable They go on to promote Diania as the immediate sister group to the states are usually assumed to have no bearing on the analysis, being arthropods, and conjecture that sclerotized and jointed limbs may reconstructed passively in the light of known states. In analyses of therefore have evolved before articulated trunk tergites in the imme- nucleotide data, by contrast, gaps may alternatively be construed as a diate arthropod stem. The data published by Liu et al.1 do not un- fifth and novel state, because shared deletions from some ancestral ambiguously support these conclusions; rather, we believe that Diania sequence may actually be informative. -

A Cambrian Unarmoured Lobopodian, †Lenisambulatrix Humboldti Gen. Et Sp
www.nature.com/scientificreports OPEN A Cambrian unarmoured lobopodian, †Lenisambulatrix humboldti gen. et sp. nov., Received: 29 January 2018 Accepted: 14 August 2018 compared with new material of Published: xx xx xxxx †Diania cactiformis Qiang Ou 1,2 & Georg Mayer2 Cambrian marine lobopodians are generally considered as predecessors of modern panarthropods (onychophorans, tardigrades, and arthropods). Hence, further study of their morphological diversity and early radiation may enhance our understanding of the ground pattern and evolutionary history of panarthropods. Here, we report a rare lobopodian species, †Lenisambulatrix humboldti gen. et sp. nov. (“Humboldt lobopodian”), from the early Cambrian Chengjiang Lagerstätte and describe new morphological features of †Diania cactiformis, a coeval armoured lobopodian nicknamed “walking cactus”. Both lobopodian species were similar in possessing rather thick, elongate lobopods without terminal claws. However, in contrast to †Diania cactiformis, the body of which was heavily armored with spines, the trunk and limbs of the Humboldt lobopodian were entirely unarmored. Our study augments the morphological diversity of Cambrian lobopodians and presents two evolutionary extremes of cuticular ornamentation: one represented by the Humboldt lobopodian, which was most likely entirely “naked”, the other epitomized by †D. cactiformis, which was highly “armoured”. Lobopodians were marine, caterpillar-like Palaeozoic animals characterized by non-segmented limbs called lobo- pods or lobopodia (singular: lobopodium; from Greek λοβός [lobos], rounded projection or protuberance; and ποδός [podos], foot). Lobopodians originated and rapidly diversifed1 during the Cambrian radiation of meta- zoan body plans and their marine representatives survived at least until the end of the Carboniferous Period2. Lobopodians are generally considered as a paraphyletic assemblage because some were most likely the forerun- ners of modern lobopod-bearing animals3–5, the onychophorans and tardigrades, and their closest relatives, the arthropods. -

Hallucigenia's Head and the Pharyngeal Armature of Early Ecdysozoans.', Nature., 523 (7558)
Durham Research Online Deposited in DRO: 14 December 2016 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Smith, M.R. and Caron, J.-B. (2015) 'Hallucigenia's head and the pharyngeal armature of early ecdysozoans.', Nature., 523 (7558). pp. 75-78. Further information on publisher's website: https://doi.org/10.1038/nature14573 Publisher's copyright statement: Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk 1 Hallucigenia’s head and the pharyngeal armature of early ecdysozoans 2 Martin R. Smith1 and Jean-Bernard Caron2,3 3 1Department of Earth Sciences, University of Cambridge, Cambridge, CB2 3EQ, UK. 4 <[email protected]> 5 2Department of Natural History (Palaeobiology Section), Royal Ontario Museum, Toronto, 6 ON M5S 2C6, Canada. <[email protected]> 7 3Department of Ecology and Evolutionary Biology and Earth Sciences, University of 8 Toronto, Toronto, ON M5S 3B2, Canada. -

Sophisticated Digestive Systems in Early Arthropods
ARTICLE Received 19 Jan 2014 | Accepted 12 Mar 2014 | Published 2 May 2014 DOI: 10.1038/ncomms4641 Sophisticated digestive systems in early arthropods Jean Vannier1,*, Jianni Liu2,*, Rudy Lerosey-Aubril1, Jakob Vinther3 & Allison C. Daley4,5 Understanding the way in which animals diversified and radiated during their early evolutionary history remains one of the most captivating of scientific challenges. Integral to this is the ‘Cambrian explosion’, which records the rapid emergence of most animal phyla, and for which the triggering and accelerating factors, whether environmental or biological, are still unclear. Here we describe exceptionally well-preserved complex digestive organs in early arthropods from the early Cambrian of China and Greenland with functional similarities to certain modern crustaceans and trace these structures through the early evolutionary lineage of fossil arthropods. These digestive structures are assumed to have allowed for more efficient digestion and metabolism, promoting carnivory and macrophagy in early arthropods via predation or scavenging. This key innovation may have been of critical importance in the radiation and ecological success of Arthropoda, which has been the most diverse and abundant invertebrate phylum since the Cambrian. 1 Universite´ Lyon 1, UMR 5276 du CNRS, Laboratoire de ge´ologie de Lyon: Terre, Plane`tes, Environnement, baˆtiment GEODE, 2, rue Raphae¨l Dubois, Villeurbanne 69622, France. 2 Early Life Institute, State Key Laboratory of Continental Dynamics, Geology Department, Northwest University, Taibai Road 229, Xi’an 710069, China. 3 University of Bristol, Departments of Earth Sciences and Biological Sciences, Woodland Road, Bristol BS8 1UG, UK. 4 Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

Sophisticated Digestive Systems in Early Arthropods Jean Vannier, Liu J., Lerosey-Aubril J., Vinther R., Daley J
Sophisticated digestive systems in early arthropods Jean Vannier, Liu J., Lerosey-Aubril J., Vinther R., Daley J. To cite this version: Jean Vannier, Liu J., Lerosey-Aubril J., Vinther R., Daley J.. Sophisticated digestive systems in early arthropods. Nature Communications, Nature Publishing Group, 2014, Sophisticated digestive systems in early arthropods, 5, pp.3641. 10.1038/ncomms4641. hal-01357174 HAL Id: hal-01357174 https://hal.archives-ouvertes.fr/hal-01357174 Submitted on 21 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License ARTICLE Received 19 Jan 2014 | Accepted 12 Mar 2014 | Published 2 May 2014 DOI: 10.1038/ncomms4641 Sophisticated digestive systems in early arthropods Jean Vannier1,*, Jianni Liu2,*, Rudy Lerosey-Aubril1, Jakob Vinther3 & Allison C. Daley4,5 Understanding the way in which animals diversified and radiated during their early evolutionary history remains one of the most captivating of scientific challenges. Integral to this is the ‘Cambrian explosion’, which records the rapid emergence of most animal phyla, and for which the triggering and accelerating factors, whether environmental or biological, are still unclear. Here we describe exceptionally well-preserved complex digestive organs in early arthropods from the early Cambrian of China and Greenland with functional similarities to certain modern crustaceans and trace these structures through the early evolutionary lineage of fossil arthropods. -
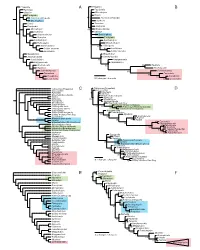
Additional IV.Pdf (168.3Kb)
Priapulida Priapulida Aysheaia A Paucipodia B Siberion Microdictyon Tardigrada Diania Antennacanthopodia Antennacanthopodia Onychophora Aysheaia Diania Xenusion Paucipodia Hadranax Microdictyon 100 Orstenotubulus Hadranax Siberion Orstenotubulus Onychophora Xenusion Tardigrada Cardiodictyon Cardiodictyon Hallucigenia Onychodictyon Onychodictyon 55 Hallucigenia Collins’ monster 88 Luolishania Luolishania Collins’ monster Megadictyon Megadictyon Jianshanopodia Jianshanopodia Pambdelurion 65 Kerygmachela Kerygmachela Pambdelurion Anomalocaris 62 62 Opabinia Opabinia Anomalocaris Schinderhannes 83 Schinderhannes Fuxianhuia Fuxianhuia 88 Eoredlichia Eoredlichia 74 Leanchoilia 0.2 changes / character 74 Leanchoilia Tubiluchus (Priapulida) Tubiluchus (Priapulida) Cricocosmia C Cricocosmia D Aysheaia Aysheaia Siberion Onychodictyon gracilis Onychodictyon gracilis Diania Hadranax Xenusion 100 61 Megadictyon Paucipodia Jianshanopodia Microdictyon Onychodictyon ferox Cardiodictyon Siberian Orsten tardigrade Hallucigenia fortis 95 60 93 64 Actinarctus (Heterotardigrada) Hallucigenia sparsa Halobiotus (Eutardigrada) Carbotubulus Kerygmachela Hallucigenia hongmeia Pambdelurion Collins’ monster Emu Bay 64 Opabinia Luolishania 90 Anomalocaris Antennacanthopodia Peytoia Ilyodes 96 69 Hurdia Euperipatoides (Onychophora) 83 Fuxianhuia 50 Chengjiangocaris Onychodictyon ferox 67 Misszhouia Siberian Orsten tardigrade 100 Kuamaia Halobiotus (Eutardigrada) Supella (Hexapoda) Actinarctus (Heterotardigrada) 81 58 Leanchoilia Hadranax 89 Alalcomenaeus Siberion Paucipodia