2020 Ecuagenera Tropical Plant List, PDF Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Temporal and Spatial Origin of Gesneriaceae in the New World Inferred from Plastid DNA Sequences
bs_bs_banner Botanical Journal of the Linnean Society, 2013, 171, 61–79. With 3 figures Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences MATHIEU PERRET1*, ALAIN CHAUTEMS1, ANDRÉA ONOFRE DE ARAUJO2 and NICOLAS SALAMIN3,4 1Conservatoire et Jardin botaniques de la Ville de Genève, Ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland 2Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Rua Santa Adélia, 166, Bairro Bangu, Santo André, Brazil 3Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland 4Swiss Institute of Bioinformatics, Quartier Sorge, CH-1015 Lausanne, Switzerland Received 15 December 2011; revised 3 July 2012; accepted for publication 18 August 2012 Gesneriaceae are represented in the New World (NW) by a major clade (c. 1000 species) currently recognized as subfamily Gesnerioideae. Radiation of this group occurred in all biomes of tropical America and was accompanied by extensive phenotypic and ecological diversification. Here we performed phylogenetic analyses using DNA sequences from three plastid loci to reconstruct the evolutionary history of Gesnerioideae and to investigate its relationship with other lineages of Gesneriaceae and Lamiales. Our molecular data confirm the inclusion of the South Pacific Coronanthereae and the Old World (OW) monotypic genus Titanotrichum in Gesnerioideae and the sister-group relationship of this subfamily to the rest of the OW Gesneriaceae. Calceolariaceae and the NW genera Peltanthera and Sanango appeared successively sister to Gesneriaceae, whereas Cubitanthus, which has been previously assigned to Gesneriaceae, is shown to be related to Linderniaceae. Based on molecular dating and biogeographical reconstruction analyses, we suggest that ancestors of Gesneriaceae originated in South America during the Late Cretaceous. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Gesneriads First Quarter 2018
GesThe Journal forn Gesneriade Growersria ds Volume 68 ~ Number 1 First Quarter 2018 Return to Table of Contents RETURN TO TABLE OF CONTENTS The Journal for Gesneriad Growers Volume 68 ~ Number 1 Gesneriads First Quarter 2018 FEATURES DEPARTMENTS 5 Saintpaulia, the NEW Streptocarpus 3 Message from the President Winston Goretsky Julie Mavity-Hudson 9 Style Guide for Writers 4 From The Editor Jeanne Katzenstein Peter Shalit 10 Gesneriads at the Liuzhou Arts Center 18 Gesneriad Registrations Wallace Wells Irina Nicholson 24 Flower Show Awards 42 Changes to Hybrid Seed List 4Q17 Paul Susi Gussie Farrice 25 Gesneriads POP in New England! 46 Coming Events Maureen Pratt Ray Coyle and Karyn Cichocki 28 62nd Annual Convention of The 47 Flower Show Roundup Gesneriad Society 51 Back to Basics: Gesneriad Crafts 37 Convention Speakers Dale Martens Dee Stewart 55 Seed Fund – Species 39 Petrocosmeas in the United Kingdom Carolyn Ripps Razvan Chisu 61 Information about The Gesneriad 43 Gasteranthus herbaceus – A white- Society, Inc. flowered Gasteranthus from the northern Andes Dale Martens with John L. Clark Cover Eucodonia ‘Adele’ grown by Eileen McGrath Back Cover and exhibited at the New York State African Petrocosmea ‘Stone Amethyst’, hybridized, Violet Convention Show, October 2017. grown, and photographed by Andy Kuang. Photo: Bob Clark See New Registrations article, page 18. Editor Business Manager The Gesneriad Society, Inc. Peter Shalit Michael A. Riley The objects of The Gesneriad [email protected] [email protected] Society are to afford -

Complete List of Gesneriad Species
Gesneriaceae Currently Aeschynanthus batakiorum Aeschynanthus jouyi Accepted Species Names Aeschynanthus batesii Aeschynanthus kermesinus Aeschynanthus brachyphyllus Aeschynanthus lancilimbus Updated 4/1/21 Aeschynanthus bracteatus Aeschynanthus lasianthus (originally SI Checklist 6-15-12 Aeschynanthus breviflorus Aeschynanthus lasiocalyx previously updated to 6/1/16) Aeschynanthus burttii Aeschynanthus lepidospermus https://padme.rbge.org.uk/grc Aeschynanthus buxifolius Aeschynanthus leptocladus Aeschynanthus calanthus Aeschynanthus leucothamnos Gesnereaceae Resource Centre - Aeschynanthus cambodiensis # Aeschynanthus ligustrinus create a checklist (rbge.org. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

Acteoside and Related Phenylethanoid Glycosides in Byblis Liniflora Salisb
Vol. 73, No. 1: 9-15, 2004 ACTA SOCIETATIS BOTANICORUM POLONIAE 9 ACTEOSIDE AND RELATED PHENYLETHANOID GLYCOSIDES IN BYBLIS LINIFLORA SALISB. PLANTS PROPAGATED IN VITRO AND ITS SYSTEMATIC SIGNIFICANCE JAN SCHLAUER1, JAROMIR BUDZIANOWSKI2, KRYSTYNA KUKU£CZANKA3, LIDIA RATAJCZAK2 1 Institute of Plant Biochemistry, University of Tübingen Corrensstr. 41, 72076 Tübingen, Germany 2 Department of Pharmaceutical Botany, University of Medical Sciences in Poznañ w. Marii Magdaleny 14, 61-861 Poznañ, Poland 3 Botanical Garden, University of Wroc³aw Sienkiewicza 23, 50-335 Wroc³aw, Poland (Received: May 30, 2003. Accepted: February 4, 2004) ABSTRACT From plantlets of Byblis liniflora Salisb. (Byblidaceae), propagated by in vitro culture, four phenylethanoid glycosides acteoside, isoacteoside, desrhamnosylacteoside and desrhamnosylisoacteoside were isolated. The presence of acteoside substantially supports a placement of the family Byblidaceae in order Scrophulariales and subclass Asteridae. Moreover, the genera containing acteoside are listed; almost all of them appear to belong to the order Scrophulariales. KEY WORDS: Byblis liniflora, Byblidaceae, Scrophulariales, chemotaxonomy, phenylethanoid gly- cosides, acteoside, in vitro propagation. INTRODUCTION et Conran, B. filifolia Planch, B. rorida Lowrie et Conran, and B. lamellata Conran et Lowrie. Byblidaceae are a small family of essentially Western Byblis liniflora Salisb. grows erect to 15-20 cm. Its lea- and Northern Australian (extending to Papuasia) herbs ves are alternate, involute in vernation, simple, linear with with exstipulate, linear sticky leaves spirally arranged a clavate apical swelling, and with stipitate, adhesive and along a more or less upright or sprawling stem and solita- sessile, digestive glands on the lamina (Huxley et al. 1992; ry, ebracteolate, pentamerous, weakly sympetalous, very Lowrie 1998). -

TKBELONSI.Pdf
UNIVERSIDADE DE SÃO PAULO FFCLRP - DEPARTAMENTO DE BIOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Palinotaxonomia em Espécies Brasileiras de Beslerieae Bartl. e Napeantheae Wiehler (Gesneriaceae) – caracteres evolutivos e influência fitogeográfica Talita Kely Belonsi Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: BIOLOGIA COMPARADA. RIBEIRÃO PRETO – SP 2018 TALITA KELY BELONSI Palinotaxonomia em Espécies Brasileiras de Beslerieae Bartl. e Napeantheae Wiehler (Gesneriaceae) – caracteres evolutivos e influência fitogeográfica Versão Original Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: BIOLOGIA COMPARADA. Orientador: Prof. Dr. Eduardo Custódio Gasparino RIBEIRÃO PRETO 2018 Autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico, para fins de estudo e pesquisa, desde que citada a fonte. Belonsi, Talita Kely Palinotaxonomia em Espécies Brasileiras de Beslerieae Bartl. Napeantheae Wiehler (Gesneriaceae) – caracteres evolutivos e influência fitogeográfica. Ribeirão Preto, 2018. 88 p.: il.; 30 cm Dissertação de Mestrado, apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto/USP. Área de concentração: Biologia Comparada. Orientador: Gasparino, Eduardo Custódio. Nome: BELONSI, Talita Kely Título: Palinotaxonomia em Espécies Brasileiras de Beslerieae Bartl. e Napeantheae Wiehler (Gesneriaceae) – caracteres evolutivos e influência fitogeográfica Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo para obtenção do título de Mestre em Ciências, área de concentração: Biologia Comparada. -

A Synoptical Classification of the Lamiales
Lamiales – Synoptical classification vers. 2.0 (in prog.) Updated: 13 December, 2005 A Synoptical Classification of the Lamiales Version 2.0 (in progress) Compiled by Richard Olmstead With the help of: D. Albach, B. Bremer, P. Cantino, C. dePamphilis, P. Garnock-Jones, R. Harley, L. McDade, E. Norman, B. Oxelman, J. Reveal, R. Scotland, J. Smith, E. Wallander, A. Weber, A. Wolfe, N. Young, M. Zjhra, and others [estimated # species in Lamiales = 22,000] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near-term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. Lamiales – Synoptical classification vers. 2.0 (in prog.) Updated: 13 December, 2005 Acanthaceae (~201/3510) Durande, Notions Elém. Bot.: 265. 1782, nom. cons. – Synopsis compiled by R. Scotland & K. Vollesen (Kew Bull. 55: 513-589. 2000); probably should include Avicenniaceae. Nelsonioideae (7/ ) Lindl. ex Pfeiff., Nomencl. -

A Publication Devoted to Tropical Plants, with Emphasis On
THE STATE OF MOLECULAR STUDIES IN THE FAMILY GESNERIACEAE: AREVIEW MICHAEL MO¨ LLER* Royal Botanic Garden Edinburgh, 20A Inverleith Row, Edinburgh EH5 3LR, Scotland, UK. Email: [email protected] JOHN L. CLARK The University of Alabama, Dept. of Biological Sciences, Box 870345, Tuscaloosa, AL 35487-0345, USA. A Publication Devoted to Tropical Plants, with Emphasis on Epiphytic Plant Families Selbyana 31(2): 95–125. 2013. THE STATE OF MOLECULAR STUDIES IN THE FAMILY GESNERIACEAE: AREVIEW MICHAEL MO¨ LLER* Royal Botanic Garden Edinburgh, 20A Inverleith Row, Edinburgh EH5 3LR, Scotland, UK. Email: [email protected] JOHN L. CLARK The University of Alabama, Dept. of Biological Sciences, Box 870345, Tuscaloosa, AL 35487-0345, USA. ABSTRACT. Sparked by the publication of large phylogenetic studies and major generic redefinitions in the Gesneriaceae, we review this growing body of molecular studies on the family. Different aspects of molecular data and their use in Gesneriaceae systematics are considered including conceptual challenges on the phylogenetic work undertaken to date as well as an overview of taxon sampling in the family. Molecular data are currently available for 70 of 72 recognized New World genera and 64 of 68 Old World genera. Many of the smaller genera and some of the larger genera are completely sampled. Current knowledge of tribal and generic delineations and relationships among the New World genera is relatively advanced. In contrast, intergeneric relationships and tribal arrangements are mostly unresolved for the Old World genera. In this paper we illustrate and summarize the published phylogenetic work in composite phylogenies with an emphasis on the most pertinent and accurate molecular systematic studies. -
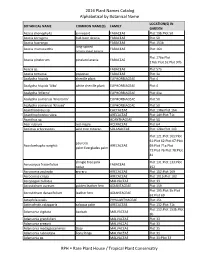
2016 Plant Names Catalog Alphabetical by Botanical Name
2016 Plant Names Catalog Alphabetical by Botanical Name LOCATION(S) IN BOTANICAL NAME COMMON NAME(S) FAMILY GARDEN Acacia choriophylla cinnecord FABACEAE Plot 19b:Plot 50 Acacia cornigera bull-horn Acacia FABACEAE Plot 50 Acacia huarango FABACEAE Plot 153b long-spined Acacia macracantha FABACEAE Plot 164 acacia:steel Acacia Plot 176a:Plot Acacia pinetorum pineland acacia FABACEAE 176b:Plot 3a:Plot 97b Acacia sp. FABACEAE Plot 57a Acacia tortuosa poponax FABACEAE Plot 3a Acalypha hispida chenille plant EUPHORBIACEAE Plot 4 Acalypha hispida 'Alba' white chenille plant EUPHORBIACEAE Plot 4 Acalypha 'Inferno' EUPHORBIACEAE Plot 41a Acalypha siamensis 'Firestorm' EUPHORBIACEAE Plot 50 Acalypha siamensis 'Kilauea' EUPHORBIACEAE Plot 50 Acanthocereus sp. CACTACEAE Plot 138a:Plot 164 Acanthophoenix rubra ARECACEAE Plot 149:Plot 71c Acanthus sp. ACANTHACEAE Plot 50 Acer rubrum red maple ACERACEAE Plot 64 Acnistus arborescens wild tree tobacco SOLANACEAE Plot 128a:Plot 143 Plot 121:Plot 161:Plot 61:Plot 62:Plot 67:Plot paurotis Acoelorrhaphe wrightii ARECACEAE 69:Plot 71a:Plot palm:Everglades palm 72:Plot 76:Plot 78:Plot 81 shingle tree:pink Plot 131:Plot 133:Plot Acrocarpus fraxinifolius FABACEAE cedar 152 Acrocomia aculeata gru-gru ARECACEAE Plot 102:Plot 169 Acrocomia crispa ARECACEAE Plot 101b:Plot 102 Acropogon bullatus MALVACEAE Plot 33 Acrostichum aureum golden leather fern ADIANTACEAE Plot 159 Plot 195:Plot 3b:Plot Acrostichum danaeifolium leather fern ADIANTACEAE 63:Plot 69 Actephila ovalis PHYLLANTHACEAE Plot 151 Actinorhytis calapparia calappa palm ARECACEAE Plot 132:Plot 71c Plot 112:Plot 153b:Plot Adansonia digitata baobab MALVACEAE 3b Adansonia grandidieri MALVACEAE Plot 33 Adansonia gregorii MALVACEAE Plot 33 Adansonia madagascariensis Bozy MALVACEAE Plot 35 Adansonia rubrostipa Fony:Ringy MALVACEAE Plot 31 Adansonia sp. -

Pollinator Adaptation and the Evolution of Floral Nectar Sugar
doi: 10.1111/jeb.12991 Pollinator adaptation and the evolution of floral nectar sugar composition S. ABRAHAMCZYK*, M. KESSLER†,D.HANLEY‡,D.N.KARGER†,M.P.J.MULLER€ †, A. C. KNAUER†,F.KELLER§, M. SCHWERDTFEGER¶ &A.M.HUMPHREYS**†† *Nees Institute for Plant Biodiversity, University of Bonn, Bonn, Germany †Institute of Systematic and Evolutionary Botany, University of Zurich, Zurich, Switzerland ‡Department of Biology, Long Island University - Post, Brookville, NY, USA §Institute of Plant Science, University of Zurich, Zurich, Switzerland ¶Albrecht-v.-Haller Institute of Plant Science, University of Goettingen, Goettingen, Germany **Department of Life Sciences, Imperial College London, Berkshire, UK ††Department of Ecology, Environment and Plant Sciences, University of Stockholm, Stockholm, Sweden Keywords: Abstract asterids; A long-standing debate concerns whether nectar sugar composition evolves fructose; as an adaptation to pollinator dietary requirements or whether it is ‘phylo- glucose; genetically constrained’. Here, we use a modelling approach to evaluate the phylogenetic conservatism; hypothesis that nectar sucrose proportion (NSP) is an adaptation to pollina- phylogenetic constraint; tors. We analyse ~ 2100 species of asterids, spanning several plant families pollination syndrome; and pollinator groups (PGs), and show that the hypothesis of adaptation sucrose. cannot be rejected: NSP evolves towards two optimal values, high NSP for specialist-pollinated and low NSP for generalist-pollinated plants. However, the inferred adaptive process is weak, suggesting that adaptation to PG only provides a partial explanation for how nectar evolves. Additional factors are therefore needed to fully explain nectar evolution, and we suggest that future studies might incorporate floral shape and size and the abiotic envi- ronment into the analytical framework. -

Gesneriaceae Currently Accepted Species Names
Gesneriaceae Currently Aeschynanthus celebica Aeschynanthus lobaticalyx Aeschynanthus ceylanicus Aeschynanthus loheri Accepted Species Names Aeschynanthus chiritoides Aeschynanthus longicaulis (Smithsonian Institution Checklist Updated) Aeschynanthus chrysanthus Aeschynanthus longiflorus Aeschynanthus citrinus Aeschynanthus macrocalyx Acanthonema diandrum Aeschynanthus copelandii Aeschynanthus madulidii Acanthonema strigosum Aeschynanthus cordifolius Aeschynanthus magnificus Aeschynanthus cordifolius Aeschynanthus mannii Achimenes admirabilis Aeschynanthus crassifolius Aeschynanthus marginatus Achimenes antirrhina Aeschynanthus cryptanthus Aeschynanthus masoniae Achimenes brevifolia Aeschynanthus cuernosensis Aeschynanthus medogensis Achimenes candida Aeschynanthus curtisii Aeschynanthus membranifolius Achimenes cettoana Aeschynanthus curvicalyx Aeschynanthus mendumiae Achimenes dulcis Aeschynanthus dasycalyx Aeschynanthus mengxingensis Achimenes erecta Aeschynanthus dempoensis Aeschynanthus meo Achimenes fimbriata Aeschynanthus dischidioides Aeschynanthus micranthus Achimenes flava Aeschynanthus dischorensis Aeschynanthus microcardia Achimenes glabrata Aeschynanthus ellipticus Aeschynanthus microphyllus Achimenes grandiflora Aeschynanthus elmeri Aeschynanthus microtrichus Achimenes heterophylla Aeschynanthus elongatus Aeschynanthus miniaceus Achimenes hintoniana Aeschynanthus everettianus Aeschynanthus miniatus Achimenes longiflora Aeschynanthus fecundus Aeschynanthus minutifolius Achimenes mexicana Aeschynanthus firmus Aeschynanthus