Monitoring Transmissibility of COVID-19 Keywords
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ningbo Facts
World Bank Public Disclosure Authorized Climate Resilient Ningbo Project Local Resilience Action Plan 213730-00 Final | June 2011 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan Contents Page 1 Executive Summary 4 2 Introduction 10 3 Urban Resilience Methodology 13 3.1 Overview 13 3.2 Approach 14 3.3 Hazard Assessment 14 3.4 City Vulnerability Assessment 15 3.5 Spatial Assessment 17 3.6 Stakeholder Engagement 17 3.7 Local Resilience Action Plan 18 4 Ningbo Hazard Assessment 19 4.1 Hazard Map 19 4.2 Temperature 21 4.3 Precipitation 27 4.4 Droughts 31 4.5 Heat Waves 32 4.6 Tropical Cyclones 33 4.7 Floods 35 4.8 Sea Level Rise 37 4.9 Ningbo Hazard Analysis Summary 42 5 Ningbo Vulnerability Assessment 45 5.1 People 45 5.2 Infrastructure 55 5.3 Economy 69 5.4 Environment 75 5.5 Government 80 6 Gap Analysis 87 6.1 Overview 87 6.2 Natural Disaster Inventory 87 6.3 Policy and Program Inventory 89 6.4 Summary 96 7 Recommendations 97 7.1 Overview 97 7.2 People 103 7.3 Infrastructure 106 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan 7.4 Economy 112 7.5 Environment 115 7.6 Government 118 7.7 Prioritized Recommendations 122 8 Conclusions 126 213730-00 | Draft 1 | 16 June 2011 110630_FINAL REPORT.DOCX World Bank Climate Resilient Ningbo Project Local Resilience Action Plan List of Tables Table -

Risk Factors for Carbapenem-Resistant Pseudomonas Aeruginosa, Zhejiang Province, China
Article DOI: https://doi.org/10.3201/eid2510.181699 Risk Factors for Carbapenem-Resistant Pseudomonas aeruginosa, Zhejiang Province, China Appendix Appendix Table. Surveillance for carbapenem-resistant Pseudomonas aeruginosa in hospitals, Zhejiang Province, China, 2015– 2017* Years Hospitals by city Level† Strain identification method‡ excluded§ Hangzhou First 17 People's Liberation Army Hospital 3A VITEK 2 Compact Hangzhou Red Cross Hospital 3A VITEK 2 Compact Hangzhou First People’s Hospital 3A MALDI-TOF MS Hangzhou Children's Hospital 3A VITEK 2 Compact Hangzhou Hospital of Chinese Traditional Hospital 3A Phoenix 100, VITEK 2 Compact Hangzhou Cancer Hospital 3A VITEK 2 Compact Xixi Hospital of Hangzhou 3A VITEK 2 Compact Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University 3A MALDI-TOF MS The Children's Hospital of Zhejiang University School of Medicine 3A MALDI-TOF MS Women's Hospital, School of Medicine, Zhejiang University 3A VITEK 2 Compact The First Affiliated Hospital of Medical School of Zhejiang University 3A MALDI-TOF MS The Second Affiliated Hospital of Zhejiang University School of 3A MALDI-TOF MS Medicine Hangzhou Second People’s Hospital 3A MALDI-TOF MS Zhejiang People's Armed Police Corps Hospital, Hangzhou 3A Phoenix 100 Xinhua Hospital of Zhejiang Province 3A VITEK 2 Compact Zhejiang Provincial People's Hospital 3A MALDI-TOF MS Zhejiang Provincial Hospital of Traditional Chinese Medicine 3A MALDI-TOF MS Tongde Hospital of Zhejiang Province 3A VITEK 2 Compact Zhejiang Hospital 3A MALDI-TOF MS Zhejiang Cancer -

Domestic and International Challenges for the Textile Industry in Shaoxing (Zhejiang)
Special feature China perspectives Domestic and International Challenges for the Textile Industry in Shaoxing (Zhejiang) SHI LU ABSTRACT: This article recounts the transformations that have taken place in the textile industry in Shaoxing, Zhejiang Province, over the course of the past 30 years. It reveals the importance of the local setup and the links that have built up between companies, mar - kets, and the state and its departments. It also exposes the difficulties experienced by companies as they try to adapt to their changing environment, whether in terms of opportunities offered by the domestic or international markets, or new regulations. KEYWORDS: textile industry, clusters, developmental state, governance, market. Introduction markets increased, the cost of labour rose, and new environmental require - ments were imposed. This contribution looks back over the transformations he developmental state concept was developed by Chalmers Johnson and the ways in which companies have adapted to these changes in the in the early 1980s to describe the role of the state in the economic case of the textile industry in Zhejiang Province and, more particularly, in successes enjoyed by Japan, which he considered to have been un - the city of Shaoxing. T (1) derestimated. It was subsequently used to refer to the ability of economic In 2013, 15% of companies in Zhejiang Province were operating in the bureaucracies to guide development in South Korea and Taiwan, channelling textile and clothing industries, making it one of China’s main centres for private investment towards growth sectors and allowing these economies manufacturing textile products at that time. (4) The city of Shaoxing, a for - to benefit from a comparative advantage in international competition. -

A Survey of Marine Coastal Litters Around Zhoushan Island, China and Their Impacts
Journal of Marine Science and Engineering Article A Survey of Marine Coastal Litters around Zhoushan Island, China and Their Impacts Xuehua Ma 1, Yi Zhou 1, Luyi Yang 1 and Jianfeng Tong 1,2,3,* 1 College of Marine Science, Shanghai Ocean University, Shanghai 201306, China; [email protected] (X.M.); [email protected] (Y.Z.); [email protected] (L.Y.) 2 National Engineering Research Center for Oceanic Fisheries, Shanghai 201306, China 3 Experimental Teaching Demonstration Center for Marine Science and Technology, Shanghai Ocean University, Shanghai 201306, China * Correspondence: [email protected] Abstract: Rapid development of the economy increased marine litter around Zhoushan Island. Social- ecological scenario studies can help to develop strategies to adapt to such change. To investigate the present situation of marine litter pollution, a stratified random sampling (StRS) method was applied to survey the distribution of marine coastal litters around Zhoushan Island. A univariate analysis of variance was conducted to access the amount of litter in different landforms that include mudflats, artificial and rocky beaches. In addition, two questionnaires were designed for local fishermen and tourists to provide social scenarios. The results showed that the distribution of litter in different landforms was significantly different, while the distribution of litter in different sampling points had no significant difference. The StRS survey showed to be a valuable method for giving a relative overview of beach litter around Zhoushan Island with less effort in a future survey. The questionnaire feedbacks helped to understand the source of marine litter and showed the impact on the local environment and economy. -

Updates on Chinese Port Information During COVID-19 Outbreak - 10.03.2020
Moir Alistair From: Harris Guy Sent: 12 March 2020 15:40 To: Group - IR Subject: FW: Huatai Info-Updates on Chinese Port Information during COVID-19 outbreak - 10.03.2020 Importance: High trProcessed: Sent From: 北京海事 <[email protected]> Sent: 10 March 2020 13:26 To: Chan Connie <[email protected]> Subject: Huatai Info-Updates on Chinese Port Information during COVID-19 outbreak. - Mar 10th, 2020 Dear Sirs/Madams, With the improvement of the epidemic situation, work resumption is taking place across China except for Hubei province, the hardest-hit region. With the increase of overseas COVID-19 cases, ports are becoming the front line of the battle against the epidemic. Most protective measures implemented by port authorities are still in force and we suggest shipowners to keep on following the notice we made in our previous Huatai Info to avoid any problems. Please note that to date our Huatai offices have come back to normal operating condition. Though some of our staff continues to work from home, they can be reached by email and mobile phone as normal. Besides the daily case handling, we shall keep collecting relevant information on COVID-19 related policy as well as latest port situation to protect Club/Members’ best interests. At the end of this Info we hereby provide the updated port information collected from local parties concerned (port authorities, survey firms, etc.) to help Club/Members make the best arrangement when your good vessel facing any potential claims during calling at Chinese ports. We really appreciate all your thoughtfulness and concern about our situation during Covid-19 epidemic. -
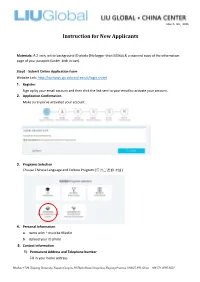
ZJU Instructions for New Applicants
March 5th, 2020 Instruction for New Applicants Materials: A 2-inch, white background ID photo (No bigger than 500kb) & a scanned copy of the information page of your passport (under 1mb in size). Step1 - Submit Online Application Form Website Link: http://isinfosys.zju.edu.cn/recruit/login.shtml 1. Register: Sign up by your email account and then click the link sent to your email to activate your account. 2. Application Confirmation Make sure you’ve activated your account. 3. Programs Selection Choose Chinese Language and Culture Program (汉语言进修项目). 4. Personal Information a. Items with * must be filled in b. Upload your ID photo 5. Contact Information 1) Permanent Address and Telephone Number Fill in your home address Mailbox 1709, Zhejiang University, Yuquan Campus, 38 Zheda Road, Hangzhou, Zhejiang Province 310027, P.R. China +86 571 8795 2051 March 5th, 2020 2) Address to Receive Admission Documents & Telephone Number a. If you are in the States, please fill in your mailing address. (please fill in the English address, otherwise it will affect the accurate postal delivery.) b. If you are out of the States, please fill in the address of China Center. (Copy the information below) Country – China City - HangZhou Postal Code – 310027 Address – Room 303, Building 11, 38 Zheda Road, Zhejiang University Yuquan Campus, Hangzhou, Zhejiang Province, P.R. China 3) Current Contacts (Copy the information below) Mailbox 1709, Zhejiang University, Yuquan Campus, 38 Zheda Road, Hangzhou, Zhejiang Province 310027, P.R. China +86 571 8795 2051 March 5th, 2020 Emergency Contact Person – Danyang (Ann) Zheng Emergency Contact Phone Number -13777886407 Emergency Contact Address - Room 303, Building 11, 38 Zheda Road, Zhejiang U niversity Yuquan Campus, Hangzhou, Zhejiang Province, P.R. -

Critical Language Scholarship Program
CRITICAL LANGUAGE SCHOLARSHIP PROGRAM Hangzhou CHINA HANDBOOK FOR PARTICIPANTS SUMMER 2014 The CLS Program is a program of the U.S. Department of State’s Bureau of Educational and Cultural Affairs. The CLS Program in China is administered by the Department of East Asian Languages and Literatures, at The Ohio State University. The Ohio State University 398 Hagerty Hall 1775 College Rd. Columbus, OH 43210-1298 Photo courtesy of Prof. Kirk Denton, Department of East Asian Languages The Ohio State University This handbook was compiled and edited by staff of the Critical Language Scholarship East Asian Languages Program at the Ohio State University and adapted from CLS handbooks from American Councils for International Education Contents Contents ......................................................................................................................................................... i Section I: Introduction .................................................................................................................................. 6 Welcome ................................................................................................................................................... 6 Fast Facts ................................................................................................................................................... 6 CLS Program ........................................................................................................................................ 6 Program Staff ....................................................................................................................................... -
![Directors and Parties Involved in the [Redacted]](https://docslib.b-cdn.net/cover/7098/directors-and-parties-involved-in-the-redacted-577098.webp)
Directors and Parties Involved in the [Redacted]
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] DIRECTORS Name Address Nationality Executive Directors Mr. Hua Bingru (華丙如) 7-6, Building 7 Chinese Jinchongfu Lanting Community Yuhang Economic Development Zone Hangzhou City, Zhejiang Province the PRC Mr. Wang Shijian (王詩劍) Room 2101, Unit 2, Building 4 Chinese Zanchengtanfu, Xinyan Road Meiyan Community Donghu Street, Yuhang District Hangzhou City, Zhejiang Province the PRC Mr. Wang Weiping (汪衛平) Room 802, Unit 3, Building 6 Chinese Junlin Tianxia City Community Shidai Community Nanyuan Street, Yuhang District Hangzhou City, Zhejiang Province the PRC Mr. Dong Zhenguo (董振國) Paiwu 39-2 Chinese Jindu Xiagong Community Maoshan Community Donghu Street, Yuhang District Hangzhou City, Zhejiang Province the PRC Mr. Xu Shijian (徐石尖) Room 702, Unit 2, Building 7 Chinese Mingshiyuan Community Renmin Dadao Baozhangqiao Community Nanyuan Street, Yuhang District Hangzhou City, Zhejiang Province the PRC –80– THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] Name Address Nationality Non-executive Directors Ms. Hua Hui (華慧) Room 101, Unit 1, Building 25 Chinese Beizhuyuan, Xiagong Huayuan 156 Beisha West Road Maoshan Community Linping Street, Yuhang District Hangzhou City, Zhejiang Province the PRC Independent non-executive Directors Mr. Yu Kefei (俞可飛) Room 1201, Unit 1, Building 5 Chinese Binjiang Huadu No. -

47030-002: Lishui River, Jinshan River
Resettlement Plan May 2015 People’s Republic of China: Jiangxi Pingxiang Integrated Rural-Urban Infrastructure Development Prepared by Shangli Project management office of the Jiangxi Pingxiang Integrated Urban and Rural Infrastructure Improvement Project for the Asian Development Bank. CURRENCY EQUIVALENTS (as of 15 May 2015) Currency unit – yuan (CNY) CNY1.00 = $0.1613 $1.00 = CNY6.2012 ABBREVIATIONS AAOV – average annual output value ADB – Asian Development Bank ADG – Anyuan District Government AHs – affected households APs – affected persons DMS – detailed measurement survey DRC – Development and Reform Committee FGD – female group discussion FSR – feasibility study report HD – house demolition HH – household IA – implementation agency JMG – Jiangxi Municipal Government LA – land acquisition LLFs – land-loss farmers LCG – Luxi County Government M&E – monitoring and evaluation MLS – minimum living security O&M – operation and maintenance PMO – Project Management Office PMG – Pingxiang Municipal Government PMTB – Pingxiang Municipal Transportation Bureau RP – resettlement plan SCG – Shangli County Government WWTP – wastewater treatment plant NOTE In this report, "$" refers to US dollars. This resettlement plan is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. -

The Ship Reporting System in Deep Water Route of Ningbo
THE SHIP REPORTING SYSTEM IN DEEP WATER ROUTE OF NINGBO-ZHOUSHAN PORT 1. Applicable Ships The Ship Reporting System is compulsory and applicable to the following types of ships which implement the “Ships’ Routeing System in Ningbo-Zhoushan Core Area”: 1.1 Passenger ships; 1.2 Ships and facilities in foreign nationality; 1.3 Dangerous cargo ships; 1.4 Ships and facilities restricted in maneuverability such as towing fleet; 1.5 Other Chinese ships of 300GT and above. 2. Applicable Geographical Area, the Number and Editions of Relevant Charts 2.1 The geographical area covered by the Ship Reporting System is the water area covering the outside door of deep-water route in Xiazhimen, Xiazhimen, Zhitouyang, Luotou waterways, Jintang waterways, Hengshuiyang, Cezi waterways, Xihoumen and so on. 2.2 The relevant charts Nautical Charts published by Maritime Safety Administration of the People’s Republic of China published, with No. of 50311, 52141, 53342, 52142, 53131 and 53132. 3. Format of Report, Content of Report and Reporting Lines 3.1 Format of Report The format for report is in accordance with the requirements by the annex of IMO Resolution A.851 (20). 3.2 Content of Report 3.2.1 General report A Ship’s name, Call Sign and IMO code (if applicable) C or D Position (latitude and longitude or position relative to the landmark) E Course F Speed G Last port of call I Port of destination O Draft Q Deficiencies and limitations (towing vessels shall report of the towing length and the name of the object being towed) DG Dangerous goods U Length Overall and Gross Tonnage 3.2.2 Ships equipped with AIS in good working condition may only need to report the following contents: A Ship’s name, Call Sign G Last port of call I Port of destination O Draft Q Deficiencies and limitations DG Dangerous goods 3.3 Reporting lines 3.3.1 Report line L1: the line connecting the Taohua Island Lighthouse and Xiazhi Island East point. -

Chapter 2 Beijing's Internal and External Challenges
CHAPTER 2 BEIJING’S INTERNAL AND EXTERNAL CHALLENGES Key Findings • The Chinese Communist Party (CCP) is facing internal and external challenges as it attempts to maintain power at home and increase its influence abroad. China’s leadership is acutely aware of these challenges and is making a concerted effort to overcome them. • The CCP perceives Western values and democracy as weaken- ing the ideological commitment to China’s socialist system of Party cadres and the broader populace, which the Party views as a fundamental threat to its rule. General Secretary Xi Jin- ping has attempted to restore the CCP’s belief in its founding values to further consolidate control over nearly all of China’s government, economy, and society. His personal ascendancy within the CCP is in contrast to the previous consensus-based model established by his predecessors. Meanwhile, his signature anticorruption campaign has contributed to bureaucratic confu- sion and paralysis while failing to resolve the endemic corrup- tion plaguing China’s governing system. • China’s current economic challenges include slowing econom- ic growth, a struggling private sector, rising debt levels, and a rapidly-aging population. Beijing’s deleveraging campaign has been a major drag on growth and disproportionately affects the private sector. Rather than attempt to energize China’s econo- my through market reforms, the policy emphasis under General Secretary Xi has shifted markedly toward state control. • Beijing views its dependence on foreign intellectual property as undermining its ambition to become a global power and a threat to its technological independence. China has accelerated its efforts to develop advanced technologies to move up the eco- nomic value chain and reduce its dependence on foreign tech- nology, which it views as both a critical economic and security vulnerability. -

The Devolution to Township Governments in Zhejiang Province*
5HGLVFRYHULQJ,QWHUJRYHUQPHQWDO5HODWLRQVDWWKH/RFDO/HYHO7KH'HYROXWLRQ WR7RZQVKLS*RYHUQPHQWVLQ=KHMLDQJ3URYLQFH -LDQ[LQJ<X/LQ/L<RQJGRQJ6KHQ &KLQD5HYLHZ9ROXPH1XPEHU-XQHSS $UWLFOH 3XEOLVKHGE\&KLQHVH8QLYHUVLW\3UHVV )RUDGGLWLRQDOLQIRUPDWLRQDERXWWKLVDUWLFOH KWWSVPXVHMKXHGXDUWLFOH Access provided by Zhejiang University (14 Jul 2016 02:57 GMT) The China Review, Vol. 16, No. 2 (June 2016), 1–26 Rediscovering Intergovernmental Relations at the Local Level: The Devolution to Township Governments in Zhejiang Province* Jianxing Yu, Lin Li, and Yongdong Shen Abstract Previous research about decentralization reform in China has primarily focused on the vertical relations between the central government and provincial governments; however, the decentralization reform within one province has not been sufficiently studied. Although the province- leading-city reform has been discussed, there is still limited research about the decentralization reform for townships. This article investigates Jianxing YU is professor in the School of Public Affairs, Zhejiang University. His current research interests include local government innovation and civil society development. Lin LI is PhD student in the School of Public Affairs, Zhejiang University. Her current research interests are local governance and intergovernmental relationships. Yongdong SHEN is postdoctoral fellow in the Department of Culture Studies and Oriental Language, University of Oslo. His current research focuses on local government adaptive governance and environmental policy implementation at the local level. Correspondence should be addressed to [email protected]. *An early draft of this article was presented at the workshop“ Greater China- Australia Dialogue on Public Administration: Maximizing the Benefits of Decen- tralization,” jointly held by Zhejiang University, Australian National University, Sun Yat-sen University, City University of Hong Kong, and National Taiwan University on 20–22 October 2014 in Hangzhou.