Thesis Effect of Additives on Laser Ignition And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Diethylenetriamine and Triethylamine Sensitization on the Critical Diameter of Nitromethane’
CP505, Shock Compression of Condensed Matter - 1999 edited by M. D. Furnish, L. C. Chhabildas, and R. S. Hixson 0 2000 American Institute of Physics l-56396-923-8/00/$17.00 EFFECT OF DIETHYLENETRIAMINE AND TRIETHYLAMINE SENSITIZATION ON THE CRITICAL DIAMETER OF NITROMETHANE’ J.J. Lee*, J. Jiang?, K.H. Choong’, J.H.S. Lee’ *Graduate Aeronautics Laboratory, California Institute of Technology, Pasadena, CA 9112.5, USA ‘Dept. of Mechanical Engineering, McGill University, Montr&al, Que’bec, Canada, H3A 2K6 In this work, the critical diameter for detonation was measured for Nitromethane (NM) sensitized with two different amines: Diethylenetriamine (DETA) and Triethylamine (TEA). The critical diameter in glass and polyvinylchloride tubes is found to decrease rapidly as the amount of sensitizer is increased, then increase past a critical amount of sensitizer. Thus the critical diameter reaches a minimum at a critical concentration of sensitizer. It was also found that the critical diameter is lower with DETA than with TEA. INTRODUCTION propagation in various tubes and channels, and the critical conditions for propagation in porous media Previous studies have shown that small (3) . concentrations of certain substances can strongly The effect of DETA on the critical diameter of increase the explosive sensitivity nitromethane NM has been reported by Engelke (4), who (NM). Amines are found to be the most effective performed measurements with up to 2.5% DETA by chemical sensitizing agent for NM with mass in NM. Engelke observed a reduction in the ethylenediamine and diethylenetriamine (DETA) critical diameter of over 50% in the range of DETA producing the largest increase in the card gap value concentrations used. -

Trihalomethanes/MTBE/Nitromethane Lab Procedure Manual
Laboratory Procedure Manual Analyte: Trihalomethanes/MTBE/Nitromethane Matrix: Whole Blood Method: Solid Phase Microextraction with GC Separation/High Resolution MS Method No: 2101.01 Revised: April 30, 2015 As performed by: Tobacco & Volatiles Branch Division of Laboratory Sciences National Center for Environmental Health Contact: Dr. Ben Blount Phone: 770-488-7894 Fax: 770-488-0181 Email: [email protected] James L. Pirkle, M.D., Ph.D. Director, Division of Laboratory Sciences Important Information for Users The Centers for Disease Control and Prevention (CDC) periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. THMs & MTBE VOCs in Blood DLS Method Code: 2101.01 National Center for Health Staistics 2 This document details the Lab Protocol for testing the items listed in the following table Data File Name Variable Name SAS Label LBXVBF Blood Bromoform (pg/mL) LBXVBM Blood Bromodichloromethane (pg/mL) VOCMWB_F LBXVCF Blood Chloroform (pg/mL) LBXVCM Blood Dibromochloromethane (pg/mL) LBXVME Blood MTBE (pg/mL) LBXVNM Blood Nitromethane (pg/mL) THMs & MTBE VOCs in Blood DLS Method Code: 2101.01 National Center for Health Staistics 3 1. Clinical Relevance and Summary of Test Principle a. Clinical Relevance The prevalence of disinfection by-products in drinking water supplies has raised concerns about possible adverse health effects from chronic exposure to these potentially carcinogenic compounds. To support studies exploring the relation between exposure to trihalomethanes (THMs), nitromethane (NM: biomarker for halonitromethanes), methyl tert-butyl ether (MTBE) and adverse health effects, an automated analytical method was developed using capillary gas chromatography (GC) and high-resolution mass spectrometry (MS) with selected ion mass detection and isotope-dilution techniques. -

Acute Tetraethyllead Poisoning
Arch. Toxikol. 24, 283--291 (1969) Acute Tetraethyllead Poisoning M. STASIK, Z. BYCZKOWSKA, S. SZENDZIKOWSKI,and Z. FIEDOttCZUK Clinical and Pathomorphological Department, Institute of Occupational Medicine, L6@, Poland, Department of Forensic Medicine, Medical Academy, L6dA l%eceived September 16, 1968 Summary. Four cases of accidental poisoning with tetraethyllead are described. Three out of four of the patients died. In the first case, pure ethyl fluid was accidentally ingested. Dominating the clinical picture of this patient were signs of greatly elevated intracranial pressure. Three other persons were poisoned as a group. They unknowingly inhaled tetra- ethyllead contained in a paint solvent they used. In these three cases, the intoxication manifested itself predominantly as a mental disorder suggestive of schizophrenia. Gross and microscopic changes observed in the fatal cases gave evidence of a capillary vascular lesion, particularly involving the vessels of the CNS. Liver damage and less severe damage to the heart muscle and kidney parenchyma were also noted. The distribution as well as the extent of the above mentioned lesions correlate approximately with the distribution and concentration of triethyllead in the various internal organs. Key- Words: Tetraethyllead -- Mental Disorder -- Damage to Parenchymatous Organs. Zusammen/assung. Die Verfasser berichten fiber 4 F/ille, yon denen 3 tSdlich waren, zuf~lliger Vergiftungen durch das sog. Ethylfluid, das Bleitetra~thyl enthElt. Im ersten Fall trat die t6dliche Vergiftung infolge irrtfimlich getrunkenem Ethylfluid auf. Als klinisches Symptom entstand erh6hter intrakranieller Druck. In den drei n~ehsten F~llen besa6 die Vergiftung einen kollektiven Charakter und war durch den Respirationstrakt zustande gekommen; zwei von den Vergifteten sind gestorben. -

FIA Technical Regulations for Drag Racing
FIA DRAG RACING SECTION 1 - JUNIOR DRAGSTER & JUNIOR FUNNY CAR 2021 Specific Regulations for FIA Drag Racing These Technical Regulations provide guidelines and minimum standards for the construction and operation of vehicles used in FIA Drag Racing. It is the responsibility of the participant to be familiar with the contents of these Technical Regulations and to comply with its requirements. It is not the responsibility of the officials to discover all potential rule compliance issues. The responsibility for compliance with these Technical Regulations rests first and foremost with the competitor. Additional safety equipment or safety-enhancing equipment is always permitted and the levels of safety equipment stated in these Technical Regulations are minimum prescribed levels for a particular type of competition and do not prohibit the individual competitor from using additional safety equipment. Competitors are encouraged to investigate the availability of additional safety devices or equipment for their type of competition. In disputed cases, whether an item, device or piece of equipment is safety-enhancing or performance-enhancing will be determined by the FIA Technical Delegate or the FIA Technical Department. Furthermore, as to performance-enhancing equipment, it is the general principle that unless optional performance-enhancing equipment or performance- related modifications are specifically permitted by these Technical Regulations, they are prohibited. Throughout these Technical Regulations, a number of references are made for particular products and equipment to meet certain standards and specifications (i.e. FIA-Standard, SFI Specs, Snell, DOT, etc.). It is important to realize that these products are manufactured to meet certainspecifications, and upon completion, the manufacturer labels the product as meeting that standard or specification. -

NMR Chemical Shifts of Common Laboratory Solvents As Trace Impurities
7512 J. Org. Chem. 1997, 62, 7512-7515 NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E. Gottlieb,* Vadim Kotlyar, and Abraham Nudelman* Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel Received June 27, 1997 In the course of the routine use of NMR as an aid for organic chemistry, a day-to-day problem is the identifica- tion of signals deriving from common contaminants (water, solvents, stabilizers, oils) in less-than-analyti- cally-pure samples. This data may be available in the literature, but the time involved in searching for it may be considerable. Another issue is the concentration dependence of chemical shifts (especially 1H); results obtained two or three decades ago usually refer to much Figure 1. Chemical shift of HDO as a function of tempera- more concentrated samples, and run at lower magnetic ture. fields, than today’s practice. 1 13 We therefore decided to collect H and C chemical dependent (vide infra). Also, any potential hydrogen- shifts of what are, in our experience, the most popular bond acceptor will tend to shift the water signal down- “extra peaks” in a variety of commonly used NMR field; this is particularly true for nonpolar solvents. In solvents, in the hope that this will be of assistance to contrast, in e.g. DMSO the water is already strongly the practicing chemist. hydrogen-bonded to the solvent, and solutes have only a negligible effect on its chemical shift. This is also true Experimental Section for D2O; the chemical shift of the residual HDO is very NMR spectra were taken in a Bruker DPX-300 instrument temperature-dependent (vide infra) but, maybe counter- (300.1 and 75.5 MHz for 1H and 13C, respectively). -

Technical Note TN-106 a Guideline for PID Instriment Response
Technical Note TN-106 05/15/VK A GUIDELINE FOR PID INSTRUMENT RESPONSE CORRECTION FACTORS AND IONIZATION ENERGIES* Example 1: RAE Systems PIDs can be used for the detection of a wide variety of With the unit calibrated to read isobutylene equivalents, the reading gases that exhibit different responses. In general, any compound with is 10 ppm with a 10.6 eV lamp. The gas being measured is butyl ionization energy (IE) lower than that of the lamp photons can acetate, which has a correction factor of 2.6. Multiplying 10 by 2.6 be measured.* The best way to calibrate a PID to different compounds gives an adjusted butyl acetate value of 26 ppm. Similarly, if the is to use a standard of the gas of interest. However, correction factors gas being measured were trichloroethylene (CF = 0.54), the adjusted have been determined that enable the user to quantify a large number value with a 10 ppm reading would be 5.4 ppm. of chemicals using only a single calibration gas, typically isobutylene. In our PIDs, correction factors can be used in one of three ways: Example 2: With the unit calibrated to read isobutylene equivalents, the reading 1. Calibrate the monitor with isobutylene in the usual fashion to is 100 ppm with a 10.6 eV lamp. The gas measured is m-xylene read in isobutylene equivalents. Manually multiply the reading (CF = 0.43). After downloading this factor, the unit should read about by the correction factor (CF) to obtain the concentration of 43 ppm when exposed to the same gas, and thus read directly in the gas being measured. -

United States Patent Office Patented Aug
2,849,302 United States Patent Office Patented Aug. 26, 1953 thasaraywarrasants, 2. east one hydrogen atom attached thereto. That is, each of the tertiary halogens is capable of forming hydro 2,849,392 gen halide with a hydrogen atom attached to a different ANTIKNoCK COMPOSITIONS carbon atom which is in a position alpha to the halogen 5 bearing carbon. In this description the term tertiary car Raymond G. Lyben, Detroit, Mich., assigner to Ethy bon atom is defined as a carbon atom which has three Corporation, New York, N. Y., a corporation of Dear other carbon atoms attached thereto by single bonds. Ware Thus, the novel scavenging agents of this invention are No Drawing. Application May 31, 955 certain chlorohydrocarbons, bromohydrocarbons and Seria Rio. 52,284 chlorobromohydrocarbons. The halohydrocarbon scav enging agents of this invention can be derived from al 7 Clains. (C. 44-69) kanes, cycloalkanes, alkenes, cycloalkenes, and hydrocar bon-substituted derivatives thereof. The smallest hydro carbon radical which can provide a scavenger of this in This invention relates to improved antiknock composi s vention contains five carbon atoms; e. g., 1,2-dihalo-1,2- tions. These compositicins encompass antiknock fluids dimethylcyclopropane. In order to provide scavengers and leaded fuels. In particular, this invention relates to having the desirable volatility characteristics with respect a class of hydrocarbons having a particular molecular to the lead antiknock agent, I prefer to employ uniform structure for use as a scavenger with lead antiknock con ly stable scavengers having up to 20 carbon atoms. pounds. Thus, it will be seen that the carbon content of my scav With the discovery of the antiknock effectiveness of or engers ranges from 5 to 20 carbons per molecule. -

Recommended Methods for the Purification of Solvents and Tests for Impurities Nitromethane
Pure & App!. Chem., Vol. 58, No. 11, pp. 1541—1545, 1986. Printed in Great Britain. © 1986 IUPAC INTERNATIONALUNION OF PURE AND APPLIED CHEMISTRY ANALYTICAL CHEMISTRY DIVISION COMMISSION ON ELECTROANALYTICAL CHEMISTRY* RECOMMENDED METHODS FOR THE PURIFICATION OF SOLVENTS AND TESTS FOR IMPURITIES NITROMETHANE Prepared for publication by J. F. COETZEE and T.-H. CHANG Department of Chemistry, University of Pittsburgh, PA 15260, USA *Membership of the Commission during 1983—85 when the report was prepared was as follows: Chairman: J. Jordan (USA); Secretary: K. Izutsu (Japan); Titular Members: A. K. Covington (UK); J. Juillard (France); R. C. Kapoor (India); E. Pungor (Hungary); Associate Members: W. Davison (UK); R. A. Durst (USA); M. Gross (France); K. M. Kadish (USA); R. Kalvoda (Czechoslovakia); H. Kao (China); Y. Marcus (Israel); T. Mussini (Italy); H. W. NUrnberg (FRG); M. Senda (Japan); N. Tanaka (Japan); K. Tóth (Hungary); National Representatives: D. D. Perrin (Australia); B. Gilbert (Belgium); W. C. Purdy (Canada); A. A. Vlek (Czecho- slovakia); H. Monien (FRG); M. L'Her (France); Gy. Farsang (Hungary); H. C. Gaur (India); W. F. Smyth (Ireland); E. Grushka (Israel); S. R. Cavallari (Italy); W. Frankvoort (Netherlands); Z. Galus (Poland); G. Johansson (Sweden); J. Buffle (Switzerland); H. Thompson (UK); J. G. Osteryoung (USA); I. Piljac (Yugoslavia). Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with JUPAC copyright symbol (© 1986 JUPAC), is printed. Publication of a translation into another language is subject to the additional condition of prior approval from the relevant JUPAC NationalAdhering Organization. Recommended methods for the purification of solvents and tests for impurities: nitromethane Themost significant solvent properties of nitromethane are discussed. -

Download Author Version (PDF)
Dalton Transactions Evaluation of Chemo- and Shape-Selective Association of a Bowl-Type Dodecavanadate Cage with an Electron-Rich Group Journal: Dalton Transactions Manuscript ID DT-ART-01-2019-000462.R1 Article Type: Paper Date Submitted by the 22-Mar-2019 Author: Complete List of Authors: Kikukawa, Yuji; Kanazawa University; JST PRESTO Kitajima, Hiromasa; Kanazawa University Hayashi, Yoshihito; Kanazawa University, Chemistry Page 1 of 6 PleaseDalton do not Transactions adjust margins Journal Name ARTICLE Evaluation of Chemo- and Shape-Selective Association of a Bowl- Type Dodecavanadate Cage with an Electron-Rich Group ab b b Received 00th January 20xx, Yuji Kikukawa, * Hiromasa Kitajima, and Yoshihito Hayashi Accepted 00th January 20xx The host-guest interaction between a half spherical-type dodecavanadate (V12) and a neutral molecule guest was evaluated DOI: 10.1039/x0xx00000x by monitoring the flip of a VO5 unit caused by the presence or absence of a guest in the cavity of V12. In N,N- www.rsc.org/ dimethylformamide (DMF), V12 adopted the guest-free-form (V12-free). By addition of several guest molecules, such as acetonitrile, nitromethane, dichloromethane, the structure conversion to the guest-inserted form (V12(guest)) were observed with the affinity constants of 137±10, 0.14±0.1, and 0.15±0.1 M−1, respectively. In the case of 1,2-dichloroethane, 1,2-dibromoethane, and 1,2-diiodoethane, the constants were 35±5, 114±5, and 2.1±0.5 M−1, respectively, suggesting that the bromo group is best fit to the cavity of the bowl. A cyclic carbonate and 5- and 6-membered lactons, cycrobutanone, and hexanal were inserted to the V12 host, while non-cyclic carbonate, non-cyclic and 7-membered cyclic ester, ketone with a 6-membered ring, and benzaldehyde showed no effect on the guest insertion. -
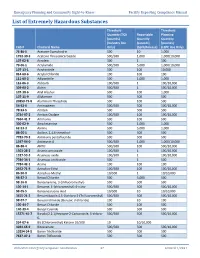
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Cumulative Cross Index to Iarc Monographs
RADIATION volume 100 D A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 2-9 June 2009 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns CUMULATIVE CROSS INDEX TO IARC MONOGRAPHS The volume, page and year of publication are given. References to corrigenda are given in parentheses. A A-α-C .............................................................40, 245 (1986); Suppl. 7, 56 (1987) Acenaphthene ........................................................................92, 35 (2010) Acepyrene ............................................................................92, 35 (2010) Acetaldehyde ........................36, 101 (1985) (corr. 42, 263); Suppl. 7, 77 (1987); 71, 319 (1999) Acetaldehyde associated with the consumption of alcoholic beverages ..............100E, 377 (2012) Acetaldehyde formylmethylhydrazone (see Gyromitrin) Acetamide .................................... 7, 197 (1974); Suppl. 7, 56, 389 (1987); 71, 1211 (1999) Acetaminophen (see Paracetamol) Aciclovir ..............................................................................76, 47 (2000) Acid mists (see Sulfuric acid and other strong inorganic acids, occupational exposures to mists and vapours from) Acridine orange ...................................................16, 145 (1978); Suppl. 7, 56 (1987) Acriflavinium chloride ..............................................13, 31 (1977); Suppl. 7, -

Tetraethyllead Is a Deadly Toxic Chemical Substance Giving Rise to Severe Psychotic Manifestations. for Its Excellent Properties
Industrial Health, 1986, 24, 139-150. Determination of Triethyllead, Diethyllead and Inorganic Lead in Urine by Atomic Absorption Spectrometry Fumio ARAI Department of Public Health St. Marianna University School of Medicine 2095 Sugao, Miyamae-ku, Kawasaki 213, Japan (Received March 10, 1986 and in revised form May 21, 1986) Abstract : A method was developed for the sequential extraction of tetraethyllead (Et4Pb), triethyllead (Et3Pb+), diethyllead (Et2Pb2+) and inorganic lead (Pb2+) from one urine sample with methyl isobutyl ketone and the subsequent sequential determination of the respective species of lead by flame and flameless atomic ab- sorption spectrometry. When 40 ml of a urine sample to which 2 ƒÊg of Pb of each of Et4Pb, Et3Pb+, Et2Pb2+ or Pb2+ had been experimentally added was assayed for the respective species of lead by flame atomic absorption spectrometry, ten repetitions of the assay gave a mean recovery rate of 98% for each of Et4Pb, Et3Pb+, and Et2Pb2+, and 99% for Pb2+, with a coefficient of variation of 2.0% for Et4Pb, 0.7% for Et3Pb+ and Pb2+, 2.6% for Et2Pb2+, and a detection limit of 4 ƒÊg of Pb/L for Et4Pb, 3 ƒÊg of Pb/L for Et3Pb+, and 5 ƒÊg of Pb/L for each of Et2Pb2+ and Pb2+. Examination of urine samples from a patient with tetraethyllead poisoning 22 days after exposure to the lead revealed that the total lead output was made up of about 51% Pb2+, about 43% Et2Pb2+, and about 6% Et3Pb+ but no Et4Pb. Ad- ministration of calcium ethylenediaminetetraacetic acid (Ca-EDTA) was followed by no increased urinary excretion of Et3Pb+ or Et2Pb2+.