Assessing the Effects of Catastrophic Fire on Plants Utilized by Two Endemic Subspecies of Spring Mountains Butterflies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Texas Prairie Dawn-Flower (Hymenoxys Texana) 5-Year Review
Texas prairie dawn-flower (Hymenoxys texana) 5-Year Review: Summary and Evaluation Photo credit: USFWS U.S. Fish and Wildlife Service Texas Coastal Ecological Services Field Office Houston, Texas Table of Contents ABBREVIATIONS ........................................................................................................................ 3 1.0 GENERAL INFORMATION .......................................................................................... 4 2.0 REVIEW ANALYSIS ...................................................................................................... 7 2.4 SYNTHESIS .................................................................................................................. 24 3.0 RESULTS....................................................................................................................... 25 4.0 RECOMMENDATIONS FOR FUTURE ACTIONS.................................................... 26 5.0 REFERENCES ............................................................................................................... 28 Appendix A ................................................................................................................................... 31 Recommendation resulting from the 5-Year Review: .................................................................. 34 Figures Figure 1 Current H. texana county occurrences ............................................................................. 9 Tables Table 1 Renaming of species historically associated with H. texana .......................................... -

Illinois Bundleflower (Desmanthus Illinoensis) Story by Alan Shadow, Manager USDA-NRCS East Texas Plant Materials Center Nacogdoches, Texas
Helping People Help The Land September/October 2011 Issue No. 11 The Reverchon Naturalist Recognizing the work of French botanist Julien Reverchon, who began collecting throughout the North Central Texas area in 1876, and all the botanists/naturalists who have followed ... Drought, Heat and Native Trees ranging from simple things like more extensive root systems, to more drastic measures like pre- Story by Bruce Kreitler mature defoliation, what they actually have little Abilene, Texas defense against is a very prolonged period of no appreciable water supply. nybody that has traveled in Texas this year A will have noticed that not only most of the By the way, even though they are usually the land browned out, but also if you look at the trees same species, there is a difference in landscape in the fields and beside the roads, they aren't trees and native trees, which are untended plants looking so good either. It doesn't take a rocket that have to fend for themselves. While they are scientist to realize that extreme high temperatures indeed the same basic trees, the differences be- combined with, and partially caused by, drought tween the environments that they live in are huge are hard on trees. and thus overall general environmental factors such as drought, temperature, and insect infesta- Since I'm pretty sure that most of the people read- tions act on them differently. For the purposes of ing this article understand very well that drought this article, I'm referring to trees that are on their is a problem for trees, the question isn't is the pre- own, untended for their entire lives in fields, pas- sent drought going to have an effect on trees, but tures, forests, or just wherever nature has placed rather, what are the present effects of the drought them and refer to them as native trees. -

Effects of Breed of Sheep and Dietary Onions on Bitterweed (Hymenoxys Odorata DC) Toxicity
Volume 29, 2014 - December Effects of Breed of Sheep and Dietary Onions on Bitterweed (Hymenoxys odorata DC) Toxicity E. S. Campbell1,3, T. R. Whitney2, C. A. Taylor, Jr.1, N. E. Garza1 1 Texas A&M AgriLife Research Center, Sonora, TX 76950 2 Texas A&M AgriLife Research and Extension Center, San Angelo, TX 76901 3 Corresponding author: [email protected] ACKNOWLEDGMENT Partial support for the research was provided by the Texas Food and Fiber Commission/ Texas Department of Agriculture, Austin, TX. Summary isonitrogenous diets consisted of alfalfa elicited a breed effect (P< 0.05) for pellets to provide 32 g DM/kg BW per serum measurements reflective of bitter- Bitterweed (Hymenoxys odorata day. Animals were group-fed onions for a weed toxicity; bilirubin, gamma-glu- DC) toxicity is a major cause of death 10-d preconditioning period, then tamyltransferase (GGT) and AST con- losses in Rambouillet sheep. This study penned and fed individually for study. centrations were greater (P ≤ 0.001) for compared the susceptibility of two Individual onion feeding commenced on DBBs than for Rambouillets. The AST, breeds [Rambouillet and Dorper × Bar- d 0 and continued through d 7. On d 3 of bilirubin, creatinine, GGT, and SUN bados Blackbelly (DBB)], of wool and onion feeding, lambs were dosed with an were clinically high for all treatments, hair sheep lambs to bitterweed toxicosis; aqueous slurry of dried bitterweed (0.25 including controls, indicating acute tox- and the potential for cull onions (Allium percent of BW, DM-basis) daily through icity. Feed refusals did not differ among cepa) to mitigate bitterweed toxicity. -

Lakeside Daisy Hymenoxys Herbacea
COSEWIC Assessment and Status Report on the Lakeside Daisy Hymenoxys herbacea in Canada THREATENED 2002 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION DES ENDANGERED WILDLIFE IN ESPÈCES EN PÉRIL CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: Please note: Persons wishing to cite data in the report should refer to the report (and cite the author(s)); persons wishing to cite the COSEWIC status will refer to the assessment (and cite COSEWIC). A production note will be provided if additional information on the status report history is required. COSEWIC 2002. COSEWIC assessment and status report the lakeside daisy Hymenoxys herbacea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 24 pp. Campbell, L.B. Husband and M.J. Oldham 2002. COSEWIC status report on the lakeside daisy Hymenoxys herbacea in Canada, in COSEWIC assessment and status report the lakeside daisy Hymenoxys herbacea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-24 pp. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’hyménoxys herbacé (Hymenoxys herbacea) au Canada Cover illustration: Lakeside Daisy — Illustration by Jack Wellington Her Majesty the Queen in Right of Canada 2003 Catalogue No. -
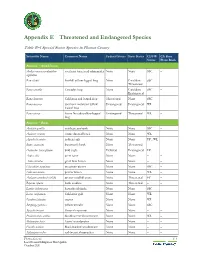
Appendix E Threatened and Endangered Species
Appendix E Threatened and Endangered Species Table E–1 Special Status Species in Plumas County Scientific Name Common Name Federal Status State Status CDFW CA Rare Status Plant Rank Animals – Amphibians Ambystoma macrodactylum southern long-toed salamander None None SSC – sigillatum Rana boylii foothill yellow-legged frog None Candidate SSC – Threatened Rana cascadae Cascades frog None Candidate SSC – Endangered Rana draytonii California red-legged frog Threatened None SSC – Rana muscosa southern mountain yellow- Endangered Endangered WL – legged frog Rana sierrae Sierra Nevada yellow-legged Endangered Threatened WL – frog Animals – Birds Accipiter gentilis northern goshawk None None SSC – Accipiter striatus sharp-shinned hawk None None WL – Aquila chrysaetos golden eagle None None FP ; WL – Buteo swainsoni Swainson's hawk None Threatened – – Haliaeetus leucocephalus bald eagle Delisted Endangered FP – Ardea alba great egret None None – – Ardea herodias great blue heron None None – – Charadrius montanus mountain plover None None SSC – Falco mexicanus prairie falcon None None WL – Antigone canadensis tabida greater sandhill crane None Threatened FP – Riparia riparia bank swallow None Threatened – – Lanius ludovicianus loggerhead shrike None None SSC – Larus californicus California gull None None WL – Pandion haliaetus osprey None None WL – Setophaga petechia yellow warbler None None SSC – Spizella breweri Brewer's sparrow None None – – Phalacrocorax auritus double-crested cormorant None None WL – Melanerpes lewis Lewis' woodpecker None -

Wyoming FWS Endangered Species List
United States Department of the Interior FISH AND WILDLIFE SERVICE Ecological Services 5353 Yellowstone Road, Suite 308A Cheyenne, Wyoming 82009 In Reply Refer To: ES-6141 1/W.32/WY07SL0361 JAN 1 '2008 Mr. Ron C. Linton Senior Groundwater Hydrologist/Project Manager U.S. Nuclear Regulatory Commission Office of Federal and State Materials And Environmental Management Programs Mail Stop T-8F5 11545 Rockville Pike Rockville, Maryland 20852-2738 Dear Mr. Linton: Please find enclosed the U.S. Fish and Wildlife Service's (Service) current list of endangered, threatened, and candidate species which may occur within the State of Wyoming. This list is provided as a general reference for the U.S. Nuclear Regulatory Commission (NRC) to use when evaluating actions under the Endangered Species Act of 1973, as amended (Act) (16 U.S.C. 1531 et seq.). We have revised our previous species list to reflect the recovery and delisting of the bald eagle (Haliaeetusleucocephalus). On July 9, 2007, the Service published a Federal Register notice (72 FR 37346) announcing that the bald eagle would be removed from the list of threatened and endangered species under the Act on August 8, 2007. However, the protections provided to the bald eagle under the Bald and Golden Eagle Protection Act, 16 U.S.C. 668 (BGEPA) and the Migratory Bird Treaty Act, 16 U.S.C. 703 (MBTA) will remain in place. The term "disturb" under the BGEPA has recently been defined as: "to agitate or bother a bald or golden eagle to a degree that causes, or is likely to cause, based on the best scientific information available, (1) injury to an eagle, (2) a decrease in its productivity, by substantially interfering with normal breeding, feeding, or sheltering behavior, or (3) nest abandonment, by substantially interfering with normal breeding, feeding, or sheltering behavior (72 FR 31332). -

160104 ARES BA Revised Per USFWS
Biological Assessment ARES Regulation Energy Management Project Prepared For: Bureau of Land Management Southern Nevada District Office 4701 North Torrey Pines Drive Las Vegas, NV 89130 BEC Environmental, Inc. Prepared By: BEC Environmental, Inc. 7660 West Sahara Avenue 7660 West Sahara Avenue, Suite 150 Suite 150 Las Vegas, Nevada 89117 Las Vegas, Nevada 89117 702.304.9830 October 2014 PO Box 11083 Revised August 2015 Reno, Nevada 89510 775.345.5261 Revised January 2016 www.becnv.com Biological Assessment for ARES REM Project August 2015 Page i of iv Table of Contents ACRONYMS ................................................................................................................... IV 1 INTRODUCTION .......................................................................................................... 1 1.1 Project Background ........................................................................................... 1 1.2 Purpose and Need ............................................................................................. 2 2 DESCRIPTION OF THE PROPOSED ACTION AND ACTION AREA .............................. 3 2.1 Proposed Action ................................................................................................. 3 2.1.1 Project Location .................................................................................................................. 4 2.1.2 Project Timing...................................................................................................................... 6 2.1.3 Project -

An Earthwise Guide for Central Texas
Native and Adapted green.org Landscape Plants City of Austin grow City of Find your perfect plant with our online seach tool! an earthwise guide for Central Texas Texas A&M AgriLife Extension Service A&M Texas Native and Adapted Landscapean earthwise Plants guide for Central Texas This guide was developed to help you in your efforts to protect and preserve our water resources. Index Key Trees ............................................................ 7 Native to: Evergreen or Deciduous: E - Edwards Plateau, Rocky, Western Zone: shallow, E – Evergreen Small Trees / Large Shrubs ........................ 9 limestone or caliche soil (generally on the west SE – Semi-evergreen side of Austin) D – Deciduous Shrubs (including roses) ............................ 15 B - Blackland Prairie, Eastern Zone: Deeper, dark, clay soils (generally on the east side of Austin) Water: Refers to the plant’s water needs during the growing Perennials .................................................. 25 B/E - Native to both Edwards Plateau and season after they are established. The majority of plants Blackland Prairie require more water while becoming established. For Austin’s current water restrictions, variances and other T - Native to Texas (not a part of Edwards Plateau or Yuccas/Agaves/Succulents/Cacti/Sotols .. 39 irrigation information visit www.WaterWiseAustin.org Blackland Prairie) VL – Very Low (Water occasionally, if no significant rain Hybrid plant with native Texas parentage Ornamental & Prairie Grasses ................... 41 X - for 30 days) For additional native plant information, visit the plant L – Low (Water thoroughly every 3-4 weeks if no Vines .......................................................... 43 section of the Lady Bird Johnson Wildflower website at significant rainfall) www.wildflower.org M – Medium (Water thoroughly every 2-3 weeks if Groundcovers ........................................... -

Water Resources Data Nevada Water Year 2003
Water Resources Data Nevada Water Year 2003 By Emil L. Stockton, Clifford Z. Jones, Ryan C. Rowland, and Rose L. Medina Water-Data Report NV-03-1 Prepared in Cooperation with the State of Nevada and with other agencies U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Gale A. Norton, Secretary U.S. Geological Survey Charles G. Groat, Director 2003 U.S. Geological Survey 333 West Nye Lane Carson City, NV 89706 (775)887-7600 Information about the USGS, Nevada District is available on the Internet at http://nevada.usgs.gov/ Information about all USGS reports and products is available by calling 1-888-ASK-USGS or on the Internet via the World Wide Web at http://www.usgs.gov/ Additional earth science information is available by accessing the USGS home page at http://www.usgs.gov/ PREFACE This report for Nevada is one of a series of annual reports that document hydrologic data gathered from the U.S. Geological Survey's surface-water and ground-water data-collection networks in each State, Puerto Rico, and the Trust Territories. These records of streams, canals, drains and springs, lakes and reservoirs, and observation wells provide the hydrologic information needed by Federal, State, and local agencies and the private sector for developing and managing our Nation's land and water resources. This report is the culmination of a concerted effort by personnel of the U.S. Geological Survey who collected, analyzed, verified, and organized the data and who typed, edited, and assembled the report. -

Guide to the Las Vegas Library Regional History Files Collection
Guide to the Las Vegas Library Regional History Files Collection This finding aid was created by Dianne Esteller, Max Gonzalez, Tammi Kim, Ani Ohanjanyan on September 25, 2017. Persistent URL for this finding aid: http://n2t.net/ark:/62930/f1zg78 © 2017 The Regents of the University of Nevada. All rights reserved. University of Nevada, Las Vegas. University Libraries. Special Collections and Archives. Box 457010 4505 S. Maryland Parkway Las Vegas, Nevada 89154-7010 [email protected] Guide to the Las Vegas Library Regional History Files Collection Table of Contents Summary Information ..................................................................................................................................... 3 Scope and Contents Note ................................................................................................................................ 3 Arrangement .................................................................................................................................................... 4 Administrative Information ............................................................................................................................. 4 Names and Subjects ........................................................................................................................................ 4 Collection Inventory ........................................................................................................................................ 5 - Page 2 - Guide to the Las Vegas -

Diversity and Origin of the Central Mexican Alpine Flora
diversity Article Diversity and Origin of the Central Mexican Alpine Flora Victor W. Steinmann 1, Libertad Arredondo-Amezcua 2, Rodrigo Alejandro Hernández-Cárdenas 3 and Yocupitzia Ramírez-Amezcua 2,* 1 Facultad de Ciencias Naturales, Universidad Autónoma de Querétaro, Av. de las Ciencias s/n, Del. Sta. Rosa Jáuregui, Querétaro 76230, Mexico; [email protected] or [email protected] 2 Private Practice, Pátzcuaro, Michoacán 61600, Mexico; [email protected] 3 Herbario Metropolitano, División de Ciencias Biológicas y de la Salud, Departamento de Biología, Universidad Autónoma Metropolitana-Iztapalapa, Avenida San Rafael Atlixco #186, Colonia Vicentina, Iztapalapa, Ciudad de México 09340, Mexico; [email protected] * Correspondence: [email protected] Abstract: Alpine vegetation is scarce in central Mexico (≈150 km2) and occurs on the 11 highest peaks of the Trans-Mexican Volcanic Belt (TMVB). Timberline occurs at (3700) 3900 m, and at 4750 m vascular plants cease to exist. The alpine vascular flora comprises 237 species from 46 families and 130 genera. Asteraceae (44), Poaceae (42), and Caryophyllaceae (21) possess 45% of the species; none of the remaining families have more than 10 species. Four species are strict endemics, and eight others are near endemics. Thirteen species are restricted to alpine vegetation but also occur outside the study area. Seventy-seven species are endemic to Mexico, 35 of which are endemic to the TMVB. In terms of biogeography, the strongest affinities are with Central or South America. Fifteen species are also native to the Old World. Size of the alpine area seems to not be the determining factor for its floristic diversity. Instead, the time since and extent of the last volcanic activity, in addition to the distance from other alpine islands, appear to be important factors affecting diversity. -

Vascular Type Specimens
University of Colorado Herbarium (COLO) 1201 Record(s) Page 1 of 322 COLO Type Specimens Acanthaceae Family: Acanthaceae Beloperone fragilis Robinson Type Status: Isotype Accession No: 422655 Bar Code: 351023 Proofed: none. Location: San Luis Potosi, Mexico. Las Canoas. Habitat: Limestone ledges. Collector: C.G. Pringle #3933. Date: 30 October, 5 December 1891. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr. Annotations: = Beloperone tenera (Rob.) Turrill. References: Proc. Amer. Acad. 27:183. 1892. Beloperone pringlei S. Watson Type Status: Isotype Accession No: 422657 Bar Code: 351031 Proofed: none. Location: Nuevo Leon, Mexico. Hills near Monterey. Habitat: none. Collector: C.G. Pringle #2548. Date: 15 July & 1 August 1889. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr. Annotations: = Justicia straminea D. Gibson. References: Proc. Amer. Acad. 25:160. 1890. Carlowrightia glandulosa Rob. & Greenm. Type Status: Isotype Accession No: 422658 Bar Code: 356568 Proofed: none. Location: Oaxaca, Mexico. Monte Alban near Oaxaca. Habitat: none. 5500 ft. Collector: C.G. Pringle #6276. Date: 5 December 1895. Miscellaneous: 1-3 feet. Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr & Frt. Annotations: none. References: Proc. Amer. Acad. 32:40. 1896. Carlowrightia ovata Gray Type Status: Isotype Accession No: 422659 Bar Code: 356576 Proofed: none. Location: Chihuahua, Mexico. Ledges near Chihuahua. Habitat: none. Collector: C.G. Pringle #932. Date: 30 August 1885. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr & Frt. Annotations: none. References: Proc. Amer. Acad. 21:406. 1886. Page 2 of 322 COLO Type Specimens Acanthaceae Carlowrightia pringlei Rob. & Greenm. Type Status: Isotype Accession No: 422660 Bar Code: 356584 Proofed: none.