Panniculitides of Particular Interest to the Rheumatologist
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Vasculitis Joseph L
Classification of Vasculitis Joseph L. Jorizzo A working classification of necrotizing vasculitis based on size of the affected vessel is proposed. The classification proposed by Gilliam and Fink in 1976 is a basis for the curren proposal. A revised working classification of vasculitis is presented. Small vessel necrotizing vasculitis and larger vessel necrotizing vasculitis categories are further subdivided. Improved understanding of the basic science aspects of vasculitis will hopefully give rise to a better consensus on the classification of vasculitis. J Invest Dermatol 100:106S–110S, 1993 A considerable portion of the now classic Medical Grand Rounds on SMALL VESSEL NECROTIZING VASCULITIS necrotizing vasculitis presented at The University of Texas Southwestern (NECROTIZING VENULITIS) Medical Center by James W. Gilliam in 1976 dealt with the classification of necrotizing vasculitis. Aspects of this presentation have been The hallmark of small-vessel necrotizing vasculitis is the diagnostic published (Table I) [1]. histopathologic finding of leukocytoclastic vasculitis including endothe- The historical context of Gilliam’s classification is important given the lial cell swelling, neutrophilic invasion of blood-vessel walls, leukocy- confounding array of classification schemas that preceded (and followed!) toclasia (neutrophilic nuclear karyorrhexis), extravasation of erythrocytes, and fibrinoid necrosis of blood vessel walls [14]. This it. The classification of vasculitis remains frustratingly controversial today. Zeek was the first to incorporate a clinicopathologic assessment process has been shown to affect post capillary venules [15,16]. This based on the size of the vessel involved in the inflammatory process in entity has been proposed as a cutaneous model for circulating immune his classification of necrotizing vasculitis in 1952 [2]. -

(ERYTHEMA NODOSUM LEPROSUM) the Term
? THE HISTOPATHOLOGY OF ACUTE PANNICULITIS NODOSA LEPROSA (ERYTHEMA NODOSUM LEPROSUM) w. J. PEPLER, M.B., Ch.B. Department of Pathology, South African Institute for Medical Research, Johannesburg R. KOOIJ, M.D. Westfort Institution, Pretoria AND J. MARSHALL, M.D. University of Pretoria, Pretoria The term "erythema nodosum leprosum" has frequently appeared in the literature on leprosy since the 1930's, apparently popularized by the Japanese (9). The occurrence of "erythema nodosum" in leprosy had previously been noted at the end of the nineteenth century by Brocq (6) and by Hansen and Looft (8), but the term seems to have fallen into disuse. As will become clear from our clinical and histological descriptions of the phenomenon commonly known as erythema nodosum leprosum, we believe the title to be a misnomer. We suggest that it would be more accurate to call it panniculitis nodosa leprosa (P.N.L,). This condition is commonly confused with acute lepra reaction, both processes being referred to as "acute reactions"; but a sharp line of distinction must be drawn between them. P.N.L. occurs only in patients with lepromatous leprosy as an acute, subacute or chronic skin eruption, with or without fever. It is characterized by showers of dusky red nodules, 0.5 to 2 cm. in diameter, on the extensor surfaces of the limbs, on the face, and, less often, on the trunk. The number of lesions appearing in an attack varies from a few to several hundreds, and they sometimes coalesce to form plaques. The nodules may be painful, and they are usually tender to touch. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
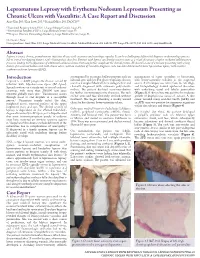
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Erythema Nodosum (En)
Buffalo Medical Group, P.C. Robert E. Kalb, M.D. Phone: (716) 630-1102 Fax: (716) 633-6507 Department of Dermatology 325 Essjay Road Williamsville, New York 14221 ERYTHEMA NODOSUM (EN) Erythema Nodosum (EN) is a relatively uncommon reaction in the skin consisting of red shiny tender nodular lesions most commonly found on the legs. The erythema refers to the red color that is seen at the surface of the skin. The nodosum refers to the fact that these lesions feel like bumps or nodules underneath the skin surface. Erythema nodosum is a reaction pattern occurring in the subcutaneous tissue and fat. It may be preceded by a low grade fever, fatigue, and joint pains. In about half of the cases, there is an internal condition occurring in the body which contributes to its formation. These include the use of certain medications, certain infections, and a number of other less likely causes. In order to identify cause for the erythema nodosum, it may be necessary to perform blood and laboratory tests. At times, however, the cause cannot be determined and erythema nodosum is considered to be idiopathic in nature. Erythema nodosum often runs an acute course lasting for a brief period from weeks to months. In a small percentage of cases, it can be a more chronic condition. The red nodules are usually confined to the lower legs, but can develop anywhere there is fat under the skin including the thighs, arms, and trunk. Initially, the lesions are more red and inflamed in appearance, but with time become more bruise like. -

MYELOPATHY ASSOCIATED with SYSTEMIC LUPUS ERYTHEMATOSUS (Erythema Nodosum)
Paraplegia 16 (1978-79) 282-294 Original Articles MYELOPATHY ASSOCIATED WITH SYSTEMIC LUPUS ERYTHEMATOSUS (Erythema Nodosum) L. S. KEWALRAMANI, M.D., M.S.Orth., S. SALEEM, M.D. and D. BERTRAND, M.T. (ASCP) Texas Institute for Rehabilitation and Research and Department of Pathology, Baylor College of Medicine, Houston, Texas 77030, U.S.A. Abstract. Two patients with sudden onset of myelopathy associated with Systemic Lupus Erythematosus (Erythema Nodosum) are described. Pertinent literature is extensively reviewed and these two new patients are added to previously reported 26 patients. Key words: Myelopathy; Meningoencephalomyelopathy; Systemic lupus erythematosus. NEUROLOGICAL manifestations of systemic lupus erythematosus (SLE) have only recently been emphasised although they were mentioned by Kaposi in 1875, who observed stupor and coma as terminal manifestations of the disease. But focal neurological abnormalities were first reported by Osler (1903) and since then there have been several reports in the literature. Most commonly reported entities have been acute organic brain syndrome, seizures and cerebrovascular disorders. Chorea, Guillain Barre syndrome, subarachnoid haemorrhage, peripheral neuro pathy and cranial nerve palsies associated with SLE have also been reported on a few occasions. Myelopathy, however, has not received adequate emphasis as a complication of SLE. Fisher and Gilmour reported the first case of flaccid paraplegia in a female with SLE in 1939. Since then only 25 additional cases have been reported in the medical literature over the past 38 years. Twenty cases have been described in sufficient detail and six briefly, to permit a meaningful review of the spinal cord involvement in this disease. We feel that there are probably many more unreported cases of myelopathy associated with SLE. -

(Tdap) Vaccine- Related Erythema Nodosum: Case Report and Review of Vaccine-Associated Erythema Nodosum
Dermatol Ther (Heidelb) (2013) 3:191–197 DOI 10.1007/s13555-013-0035-9 CASE REPORT Combined Reduced-Antigen Content Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccine- Related Erythema Nodosum: Case Report and Review of Vaccine-Associated Erythema Nodosum Philip R. Cohen To view enhanced content go to www.dermtherapy-open.com Received: September 23, 2013 / Published online: November 1, 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com ABSTRACT literature search was performed on erythema nodosum, vaccine, and vaccination. Background: Vaccination programs reduce the Results: Tdap, the most commonly used booster morbidity and mortality of diphtheria, vaccine against tetanus, diphtheria, and pertussis, pertussis, and tetanus. Erythema nodosum is a is well tolerated in all age groups. Local injection- reactive erythema that can be associated with site reactions are the most common adverse events, infections, drugs, and many conditions. The whereas headache, fatigue, gastrointestinal new onset of erythema nodosum after receiving symptoms, and fever are the most frequent vaccination is uncommon. systemic events. Erythema nodosum has not Purpose: Combined reduced-antigen content previously been reported in patients who have tetanus, diphtheria, and acellular pertussis received Tdap vaccine. The patient developed (Tdap) vaccine-associated erythema nodosum erythema nodosum within 48 h after receiving is described and the reports of vaccine-related Tdap vaccine; her symptoms cleared and nearly all erythema nodosum are summarized. skin lesions resolved within 2 weeks after initiating Methods: The clinical features of a 39-year-old oral treatment with ibuprofen, fexofenadine, and woman who developed erythema nodosum prednisone. Vaccine-associated erythema after receiving Tdap vaccine are reported. -

Cutaneous Sarcoidosis: a Dermatologic Masquerader RAJANI KATTA, M.D., Baylor College of Medicine, Houston, Texas
Cutaneous Sarcoidosis: A Dermatologic Masquerader RAJANI KATTA, M.D., Baylor College of Medicine, Houston, Texas Sarcoidosis is a multisystem disease that may involve almost any organ system; therefore, it results in various clinical manifestations. Cutaneous sarcoidosis occurs in up to one third of patients with systemic sarcoidosis. Recognition of cutaneous lesions is important because they provide a visible clue to the diagnosis and are an easily accessible source of tissue for histologic examination. Because lesions can exhibit many different morpholo- gies, cutaneous sarcoidosis is known as one of the “great imitators” in dermatology. Spe- cific manifestations include papules, plaques, lupus pernio, scar sarcoidosis, and rare mor- phologies such as alopecia, ulcers, hypopigmented patches, and ichthyosis. Treatment of cutaneous lesions can be frustrating. For patients with severe lesions or widespread involvement, the most effective treatment is systemic glucocorticoids. (Am Fam Physician 2002;65:1581-4. Copyright© 2002 American Academy of Family Physicians.) arcoidosis is a systemic disease that with sarcoidosis when a compatible clinical or can involve almost any organ sys- radiologic picture is present, along with his- tem. Infiltration with noncaseating tologic evidence of noncaseating granulomas, granulomas is the hallmark of the and when other potential causes, such as disease, and it may result in various infections, are excluded.1 Sclinical manifestations. The underlying cause of sarcoidosis remains unknown.1 Although Recognition of Skin Lesions the disease can occur at any age, in persons of Recognition of cutaneous lesions is impor- either gender, and in all races, older studies tant because they provide a visible clue to the suggest that sarcoidosis more frequently diagnosis and are an easily accessible source affects persons who are of Scandinavian, of tissue for histologic examination. -

Bazin's Disease (Erythema Induratum)
Images in Rheumatology Clinical Images: Bazin’s Disease (Erythema Induratum) MANAL AL-MASHALEH, MD, JBM, Visiting Fellow, Rheumatology Department; DON PACKHAM, MBBS, FRACP, Staff Specialist, Infectious Disease Department, Westmead Hospital; NICHOLAS MANOLIOS, MBBS(Hons), MD, PhD, FRACP, FRCPA, Director of Rheumatology, Associate Professor, University of Sydney, Rheumatology Department, Westmead Hospital, Sydney, Australia. Address reprint requests to Dr. Manolios. E-mail: [email protected] Our case highlights the similarity between erythema nodosum Bazin’s disease (EI) is an under-recognized chronic recur- (EN) and erythema induratum (EI) and illustrates the impor- rent condition characterized by painless, deep-seated, subcuta- tance of Mantoux testing in investigations of patients with neous induration, which gradually extends to the skin surface, vasculitis, particularly those from tuberculous-endemic areas; forming bluish-red nodules or plaques, which then often ulcer- as well, it points to the need for biopsy if apparent EN has ate1,2. The morphologic, molecular, and clinical data suggest atypical or prolonged course or is complicated by ulceration, that EI represents a hypersensitivity reaction to tubercle bacil- and the resolution of EI with anti-TB treatment alone. lus3. As described, it is not unusual to have negative cultures A 16-year-old Indonesian girl with a 2 year history of and fail to detect M. tuberculosis by PCR amplification2,4. Sjögren’s syndrome (SSA/SSB-positive) and hepatitis C and taking no medications presented with a 2 week history of painful REFERENCES erythematous nodules over the anterior aspect of her lower limbs 1. Bayer-Garner IB, Cox MD, Scott MA, Smoller BR. -

A Case Report of Chronic Sclerosing Panniculitis Hadiuzzaman*, M
Journal of Pakistan Association of Dermatologists 2010; 20 : 246-248. Case Report A case report of chronic sclerosing panniculitis Hadiuzzaman*, M. Hasibur Rahman*, Nazma Parvin Ansari**, Aminul Islam† *Department of Dermatology, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh. **Department of Pathology, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh †Department of Medicine, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh Abstract Sclerosing panniculitis is a fibrotic process that usually occurs on the legs, commonly in women older than 40. The principal features are indurated woody plaques with erythema, edema, telangiectasia, and hyperpigmentation. Although the exact pathogenesis is uncertain, it is thought to occur as a result of ischemic changes. We present a 28-year-old married female who had a 10- year history of painful sclerotic plaques, repeated ulceration and healing with fibrosis of the both lower legs and abdomen. Venogram and Doppler investigations were normal. Skin biopsy from the edge of the ulcer demonstrated the feature of chronic sclerosing panniculitis. Satisfactory improvement was found with methotrexate 7.5mg weekly for 4 months. No recurrence was noted within 1 year follow up. Key words Sclerosing panniculitis, lipodermatosclerosis. Case report Mild swelling of the legs worse at the end of the day was also reported. Tenderness of the ulcer A 28-year-old married female presented to was worse with dependency. There was no dermatology outpatient, Community Based history of previous trauma to the area, joint Medical College, Bangladesh, with a 10-year complaint, pancreatic disease, or other tender history of painful repeated ulceration and nodular lesions or ulcerations. There was no healing with fibrosis of the both lower legs and significant history of fever and night sweating. -

Sclerema Neonatorum Treated with Intravenous Immunoglobulin: a Case Report and Review of Treatments
Sclerema Neonatorum Treated With Intravenous Immunoglobulin: A Case Report and Review of Treatments Kesha J. Buster, MD; Holly N. Burford, MD; Faith A. Stewart, MD; Klaus Sellheyer, MD; Lauren C. Hughey, MD Practice Points Sclerema neonatorum is a rare neonatal panniculitis with a high mortality rate. Exchange transfusion improves survival, but its use in neonates has declined. Intravenous immunoglobulin represents a novel treatment option that may lead to increased survival in pre- term newborns with sclerema neonatorum. Sclerema neonatorum (SN)CUTIS is a rare neonatal improvement. Sclerema neonatorum remains a panniculitis that typically develops in severely poorly understood and difficult to treat neona- ill, preterm newborns within the first week of tal disorder. Although IVIG did not prevent our life and often is fatal. It usually occurs in pre- patient’s death, further studies are needed to term newborns with delivery complications such determine its clinical utility in the treatment of this as respiratory distress or maternal complica- rare disorder. tions such as eclampsia. Few clinical trials have Cutis. 2013;92:83-87. beenDo performed to address Notpotential treatments. Copy Successful treatment has been achieved via exchange transfusion (ET), but its use in neonates clerema neonatorum (SN) is a rare neonatal is declining. Similar to ET, intravenous immuno- panniculitis that typically develops in severely globulin (IVIG) enhances both humoral and Sill, preterm newborns within the first week cellular immunity and thus may decrease mor- of life. It is characterized by rapidly progressive tality associated with SN. We report a case of induration of subcutaneous fat. Treatments include SN in a term newborn who subsequently devel- supportive care, emollients, warming/maintaining oped septicemia.