Panniculitis, a Rare Presentation of Onset and Exacerbation of Juvenile Dermatomyositis: a Case Report and Literature Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cutaneous Sarcoidosis: a Dermatologic Masquerader RAJANI KATTA, M.D., Baylor College of Medicine, Houston, Texas
Cutaneous Sarcoidosis: A Dermatologic Masquerader RAJANI KATTA, M.D., Baylor College of Medicine, Houston, Texas Sarcoidosis is a multisystem disease that may involve almost any organ system; therefore, it results in various clinical manifestations. Cutaneous sarcoidosis occurs in up to one third of patients with systemic sarcoidosis. Recognition of cutaneous lesions is important because they provide a visible clue to the diagnosis and are an easily accessible source of tissue for histologic examination. Because lesions can exhibit many different morpholo- gies, cutaneous sarcoidosis is known as one of the “great imitators” in dermatology. Spe- cific manifestations include papules, plaques, lupus pernio, scar sarcoidosis, and rare mor- phologies such as alopecia, ulcers, hypopigmented patches, and ichthyosis. Treatment of cutaneous lesions can be frustrating. For patients with severe lesions or widespread involvement, the most effective treatment is systemic glucocorticoids. (Am Fam Physician 2002;65:1581-4. Copyright© 2002 American Academy of Family Physicians.) arcoidosis is a systemic disease that with sarcoidosis when a compatible clinical or can involve almost any organ sys- radiologic picture is present, along with his- tem. Infiltration with noncaseating tologic evidence of noncaseating granulomas, granulomas is the hallmark of the and when other potential causes, such as disease, and it may result in various infections, are excluded.1 Sclinical manifestations. The underlying cause of sarcoidosis remains unknown.1 Although Recognition of Skin Lesions the disease can occur at any age, in persons of Recognition of cutaneous lesions is impor- either gender, and in all races, older studies tant because they provide a visible clue to the suggest that sarcoidosis more frequently diagnosis and are an easily accessible source affects persons who are of Scandinavian, of tissue for histologic examination. -

A Case Report of Chronic Sclerosing Panniculitis Hadiuzzaman*, M
Journal of Pakistan Association of Dermatologists 2010; 20 : 246-248. Case Report A case report of chronic sclerosing panniculitis Hadiuzzaman*, M. Hasibur Rahman*, Nazma Parvin Ansari**, Aminul Islam† *Department of Dermatology, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh. **Department of Pathology, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh †Department of Medicine, Community Based Medical College, Bangladesh, Mymensingh, Bangladesh Abstract Sclerosing panniculitis is a fibrotic process that usually occurs on the legs, commonly in women older than 40. The principal features are indurated woody plaques with erythema, edema, telangiectasia, and hyperpigmentation. Although the exact pathogenesis is uncertain, it is thought to occur as a result of ischemic changes. We present a 28-year-old married female who had a 10- year history of painful sclerotic plaques, repeated ulceration and healing with fibrosis of the both lower legs and abdomen. Venogram and Doppler investigations were normal. Skin biopsy from the edge of the ulcer demonstrated the feature of chronic sclerosing panniculitis. Satisfactory improvement was found with methotrexate 7.5mg weekly for 4 months. No recurrence was noted within 1 year follow up. Key words Sclerosing panniculitis, lipodermatosclerosis. Case report Mild swelling of the legs worse at the end of the day was also reported. Tenderness of the ulcer A 28-year-old married female presented to was worse with dependency. There was no dermatology outpatient, Community Based history of previous trauma to the area, joint Medical College, Bangladesh, with a 10-year complaint, pancreatic disease, or other tender history of painful repeated ulceration and nodular lesions or ulcerations. There was no healing with fibrosis of the both lower legs and significant history of fever and night sweating. -

Sclerema Neonatorum Treated with Intravenous Immunoglobulin: a Case Report and Review of Treatments
Sclerema Neonatorum Treated With Intravenous Immunoglobulin: A Case Report and Review of Treatments Kesha J. Buster, MD; Holly N. Burford, MD; Faith A. Stewart, MD; Klaus Sellheyer, MD; Lauren C. Hughey, MD Practice Points Sclerema neonatorum is a rare neonatal panniculitis with a high mortality rate. Exchange transfusion improves survival, but its use in neonates has declined. Intravenous immunoglobulin represents a novel treatment option that may lead to increased survival in pre- term newborns with sclerema neonatorum. Sclerema neonatorum (SN)CUTIS is a rare neonatal improvement. Sclerema neonatorum remains a panniculitis that typically develops in severely poorly understood and difficult to treat neona- ill, preterm newborns within the first week of tal disorder. Although IVIG did not prevent our life and often is fatal. It usually occurs in pre- patient’s death, further studies are needed to term newborns with delivery complications such determine its clinical utility in the treatment of this as respiratory distress or maternal complica- rare disorder. tions such as eclampsia. Few clinical trials have Cutis. 2013;92:83-87. beenDo performed to address Notpotential treatments. Copy Successful treatment has been achieved via exchange transfusion (ET), but its use in neonates clerema neonatorum (SN) is a rare neonatal is declining. Similar to ET, intravenous immuno- panniculitis that typically develops in severely globulin (IVIG) enhances both humoral and Sill, preterm newborns within the first week cellular immunity and thus may decrease mor- of life. It is characterized by rapidly progressive tality associated with SN. We report a case of induration of subcutaneous fat. Treatments include SN in a term newborn who subsequently devel- supportive care, emollients, warming/maintaining oped septicemia. -

CUTANEOUS SARCOIDOSIS by GORDON B
274 Postgrad Med J: first published as 10.1136/pgmj.34.391.274 on 1 May 1958. Downloaded from , II CUTANEOUS SARCOIDOSIS By GORDON B. MITCHELL-HEGGS, M.D., F.R.C.P. and MICHAEL FEIWEL, M.B., Ch.B., M.R.C.P. Department of Dermatology, St. Mary's Hospital, W.2 Sarcoidosis of the skin is often a striking picture for systemic features, a skin biopsy is again an easy and led to its recognition as a disease entity. For means of establishing the diagnosis. the patient, its importance lies in disfigurement In either case, the clinician is helped if he carries more than in disability. For the clinician, it may in his mind's eye the varying aspects of cutaneous provide a ready means of diagnosis towards which sarcoidosis. At the same time, conditions re- one glance may give a clue. In addition, the skin sembling sarcoidosis of the skin must be differ- has played an important role in the study of entiated. This is not easy because the eye needs aetiology. The reactions to injected tuberculin, practice and neither description nor photograph the response to B.C.G. inoculation, and to Kveim can adequately convey the subtleties of the make- antigen are some of the ways in which the skin has up of a skin lesion on which a diagnosis rests. been tested in sarcoidosis. Clinical Manifestations Sarcoidosis The picture of the skin is a varied one and classi- The aetiology is not definitely established. The fication based on the early descriptions is into four disorder involves the reticulo-endothelial system types: Boeck's sarcoid, subcutaneous sarcoid ofcopyright. -

Cold Panniculitis Neonatorum
I M A G E S Cold Panniculitis Neonatorum R G HOLLA AND *AMARENDRA NARAYAN PRASAD Military Hospital, 166 MH, Jammu; and *Department of Pediatrics, Military Hospital, Namkum, Ranchi 834 010, India. E-mail : [email protected] ocalised areas of erythema and induration play a role in its causation. The eruptive phase usu- developed on the feet of 2 term neonates ally begins 48 (6-72) hours after a cold injury to ex- (male and a female) on the 7th and 10th posed or poorly protected areas. Lesions present as Lday of life respectively, at the peak of win- localized indurated nodules with ill-defined margins. ters in the plains of North Nodules are firm or hard India. There were no pre- and cold and painful. ceding perinatal risk fac- Cutaneous distribution tors or complications. in children characteristi- The babies had no direct cally is on the face exposure to any cold ob- (cheeks and forehead) ject or ice. Woody and extremities (feet and erythema was noted first, hand). Cold panniculitis followed by (24 to 48 neonatorum should be hrs) formation of red- differentiated from purple nodules. Gradual sclerema neonatorum, rewarming was done poststeroid panniculitis over a period of days, and and chill blains. Biopsy both babies had complete is reserved for diagnos- recovery. tic problem cases. The Cold panniculitis classic features of cold neonatorum, also called panniculitis on histopa- adiponecrosis subcu- thology predominantly tanea is an acute, nodu- are a lobular panniculi- lar, erythematous erup- tis with scattered tion usually limited to ar- lympho histiocytic and eas exposed to the cold in eosinophilic infiltrates. -

An Atypical Presentation of Erythema Induratum
Letters to Editor Address for correspondence: Wg Cdr Sandeep Arora, Skin diagnosis of EN. Skin biopsy from a leg nodule revealed Department, 5 AFH, c/o 99 APO, India. lobular panniculitis with caseous necrosis, epithelioid cell E-mail: [email protected] granulomas, and Langhans giant cells [Figure 1]. Small REFERENCES vessel vasculitis with extravasation of RBCs, fibrinoid degeneration of vessel wall, and karyorrhexis were also 1. Saoji VA. Hand, foot and mouth disease in Nagpur. Indian J present. The histopathology was consistent with EI. Dermatol Venereol Leprol 2008;74:133-5. Findings from her hemogram, liver and renal function 2. Frydenberg A, Starr M. Hand, foot and mouth disease. Aust tests, urine and stool examination, anti-nuclear antibody, Fam Physician 2003;32:594-5. anti-streptolysin O titer were within normal limits. Chest 3. Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, et x-ray was normal, but findings from Mantoux test was al. Clinical features and risk factors of pulmonary edema strongly positive (45 mm induration with necrosis). after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999;354:1682-6. 4. Podin Y, Gias EL, Ong F, Leong YW, Yee SF, Yusof MA, et al. Based on these findings, we initiated four-drug antitubercular Sentinel surveillance for human enterovirus 71 in Sarawak, therapy (ATT). Within 3 weeks, the lesions resolved and no Malaysia: lessons from the first 7 years. BMC Public Health new lesions appeared. ATT was continued for 6 months. 2006;6:180. 5. Sasidharan CK, Sugathan P, Agarwal R, Khare S, Lal S, The clinical manifestation of recurrent tender subcutaneous Jayaram Paniker CK. -

Subcutaneous Fat Necrosis of Newborn - a Rare Case Report of Lobular Panniculitis in a Neonate
IP Indian Journal of Clinical and Experimental Dermatology 2021;7(3):266–269 Content available at: https://www.ipinnovative.com/open-access-journals IP Indian Journal of Clinical and Experimental Dermatology Journal homepage: www.ijced.org/ Case Report Subcutaneous fat necrosis of newborn - A rare case report of lobular panniculitis in a neonate Samagani Akshay1,*, Pemmanda Raju Belliappa1, Raveendra Leena1 1Dept. of Dermatology, Venereology & Leprosy, RajaRajeswari Medical College & Hospital, Kambipura, Karnataka, India ARTICLEINFO ABSTRACT Article history: Subcutaneous fat necrosis of newborn is a rare cutaneous disorder affecting neonates. It usually presents Received 07-06-2021 as subcutaneous nodules or plaques, within the first few weeks of life, following an eventful delivery. It is Accepted 26-07-2021 characterized by hypercalcemia, which may present with lethargy, irritability, hypotonia and dehydration, Available online 04-09-2021 mimicking sepsis. Histopathology is proven to be the gold standard in diagnosis with characteristic lobular panniculitis, mixed inflammatory cell infiltrate and radially arranged crystals. This needs to be differentiated from other causes of lobular panniculitis, as early diagnosis and treatment to prevent Keywords: long-term complications are advocated. Education of parents regarding the disease and danger signs of subcutaneous fat necrosis of newborn hypercalcemia and weekly monitoring of serum calcium is recommended. Treatment based on rehydration, perinatal asphyxia dietary vitamin D and calcium restriction, Furosemide and prednisolone are considered. We have discussed hypercalcemia a case of subcutaneous fat necrosis, in an 8-week-old male baby. lobular panniculitis Key Messages: Subcutaneous fat necrosis is an important differential in neonates presenting with palpable neonatal sepsis subcutaneous nodules, along with sclerema neonatorum. -

Sarcoid-Like Reaction in a Patient Recovering from Coronavirus Disease 19 Pneumonia
University of Massachusetts Medical School eScholarship@UMMS COVID-19 Publications by UMMS Authors 2020-07-24 Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia Sara Behbahani Rutgers University - Newark Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/covid19 Part of the Dermatology Commons, Infectious Disease Commons, Medical Immunology Commons, Pathological Conditions, Signs and Symptoms Commons, Skin and Connective Tissue Diseases Commons, and the Virus Diseases Commons Repository Citation Behbahani S, Baltz J, Droms R, Deng AC, Amano SU, Levin NA, O'Brien MC, Wiss K. (2020). Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia. COVID-19 Publications by UMMS Authors. https://doi.org/10.1016/j.jdcr.2020.07.026. Retrieved from https://escholarship.umassmed.edu/covid19/101 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in COVID-19 Publications by UMMS Authors by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. CASE REPORT Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia Sara Behbahani, MS,a JuliaO.Baltz,MD,b,c Rebecca Droms, MD,b AprilC.Deng,MD,d Shinya U. Amano, MD, PhD,d NikkiA.Levin,MD,PhD,b Mary Callery O’Brien, MD,e and Karen Wiss, MDb,f Newark, New Jersey; Worcester, Massachusetts; East Greenwich, Rhode Island Key words: coronavirus disease 2019; COVID-19; dermatologic manifestations of disease; sarcoidosis; sarcoid- like reactions; SARS-CoV-2; severe acute respiratory syndrome coronavirus 2. -

Inherited Immunodeficiency
Inherited Immunodeficiency: Brigitte Bader-Meunier, MD, a, b Frédéric Rieux-Laucat, PhD, b Fabien Touzot, MD, PhD, c Marie-Louise Frémond, MD, a, b AIsabelle New André-Schmutz, Association PhD, b Sylvie Fraitag, MD, d Christine With Bodemer, MD, PhDEarly-b, e, f Onset Childhood Panniculitis abstract We report on 4 children who presented with asepticTRNT1 panniculitisNF- κb2 associated with inherited immunodeficiency. Three patients had a B-cell immunodeficiency resulting from mutations in the and genes aDépartements d'Immunologie, Hématologie, et LCK Rhumatologie Pédiatrique, dd'Anatomie Pathologique, (no mutation was found in the third patient), and 1 had a T-cell deficiency and ede Dermatologie Pédiatrique, Hôpital Necker-Enfants (mutation in the gene). Panniculitis occurred before the age of 2 years Malades, Assistance Publique-Hôpitaux de Paris, Paris, b f in the 4 patients and preceded the onset of recurrent infections because France; Institut Imagine, INSERM U1163; Université Paris Descartes, Sorbonne Cité, Paris, France; and cDépartement of immunodeficiency in 2. It presented either as nodules, which resolved d’Immunologie et Rhumatologie Pédiatrique, Centre spontaneously within 1 to 2 weeks (3 patients), or chronic ulcerative lesions Hospitalier Universitaire Sainte-Justine, Université de Montréal, Quebec, Canada (1 patient) associated with unexplained fever and elevated acute phase Drs Bodemer, Fraitag, and Bader-Meunier reactants, without evidence of infection or high-titer autoantibodies. Febrile conceptualized and designed -

Pancreatic Panniculitis Secondary to Acinar Cell Carcinoma of the Pancreas
Pancreatic Panniculitis Secondary to Acinar Cell Carcinoma of the Pancreas Ian R. Gorovoy, MD; John McSorley, MD; Jaclyn B. Gorovoy, BS Practice Points Panniculitis frequently represents a dermatologic manifestation of a systemic disease and therefore mer- its consideration of a systemic workup. Pancreatic panniculitis can occur in the setting of normal pancreatic enzyme levels. Pathologic features of pancreatic panniculitis include a predominantly lobular panniculitis without vascu- litis, necrotic adipocytes called ghost cells, and calcium salts deposited within the adipocyte cytoplasm. The triad of panniculitis, recent weight loss, and new-onset depression is particularly concerning for pancreatic cancer. We report the case of a 68-year-old white woman progressively developed and worsened over the last who presented with painful, CUTIS1- to 4-cm, erythema- month despite treatment with cephalexin prescribed tous nodules located bilaterally on the anterior by her primary care physician (Figure 1). Her medi- and medial shins that had progressively devel- cal history was remarkable for non–small cell lung oped and worsened over the last month. Workup cancer that currently was in remission after treat- revealed pancreatic panniculitis (PP) secondary ment with radiation and chemotherapy, as well as to acinar cell carcinoma of the pancreas (ACCP). diabetes mellitus, chronic anemia, nephrolithiasis, The unique clinicopathologic features, differen- gastroesophageal reflux disease, migraines, hyperlip- tial Dodiagnosis, underlying Notcauses, associated idemia, Copy and fibromyalgia. laboratory and clinical findings, pathophysiology, A review of systems was positive for 10-lb unin- treatments, and appropriate workup for PP also tentional weight loss in the last 6 months, increased are reviewed. arthralgias in the last 2 months primarily involving Cutis. -

Generalized Lipodystrophy (AGL; Lawrence Syndrome)
Acquired: Generalized Lipodystrophy (AGL; Lawrence Syndrome) This is a rare disease characterized by generalized disappearance of fat occurring during childhood and adolescence. A total of about 80 cases have been reported. Patients have normal body fat at birth thus distinguishing them from patients with congenital generalized lipodystrophy. Females are affected approximately 3 times more often than males. AGL could be due to panniculitis (inflammation in the fat) or autoimmune diseases, however, in several cases no causative factor could be identified. The onset of AGL may also occur following infections such as varicella, measles, pertussis, diphtheria, pneumonia, osteomyelitis, parotitis, infectious mononucleosis, and hepatitis. Usually, the onset of lipodystrophy is gradual over months to years. Eventually generalized and near complete loss of fat may occur in some patients resulting in muscular appearance and prominent superficial veins (veins under the skin). There is loss of fat from the palms and soles also, while retroorbital and bone marrow fat are preserved. The degree of loss of intraabdominal fat varies. Affected children may have a voracious appetite. Dark velvety pigmentation (acanthosis nigricans) may occur in the axilla and neck, umbilicus, nipples and occasionally hands and feet. Increased linear growth may be seen in the children. Excess body hair, enlargement of genitalia (clitoromegaly) and occasional ovarian cysts (enlarged ovaries) may be seen in the females. Enlargement of liver and spleen is frequently seen. Cirrhosis has been reported in about one fifth of the patients as a late sequela of hepatic steatosis or autoimmune hepatitis. The patients commonly have moderately severe elevation of blood lipids. Diabetes occurs usually after the onset of lipodystrophy. -
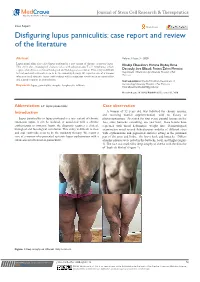
Disfiguring Lupus Panniculitis: Case Report and Review of the Literature
Journal of Stem Cell Research & Therapeutics Case Report Open Access Disfiguring lupus panniculitis: case report and review of the literature Abstract Volume 6 Issue 3 - 2020 Lupus panniculitis also called lupus profound is a rare variant of chronic cutaneous lupus. Khadija Elboukhari, Hanane Baybay, Rime This entity share histological characteristics with subcutaneous T cell lymphoma, which require a lot of times, a clinical biological and histological correlation. This entity is difficult Dassouly, Sara Elloudi, Fatima Zahra Mernissi Department of Dermatology, University Hospital of Fez, to treat and oral corticoids seem to be the mainstay therapy. We report a case of a woman Morocco who presented systemic lupus erythematous with a cutaneous involvement as panniculitis and a good response to antimalarials. Correspondence: Khadija Elboukhari, Department of Dermatology, University Hospital of Fez, Morocco, Keywords: lupus, panniculitis, atrophy, lymphocytic infiltrate Email Received: June 28, 2020 | Published: December 31, 2020 Abbreviation: LP: lupus panniculitis Case observation Introduction A woman of 52 years old, was followed for chronic anemia, and receiving martial supplementation, with no history of Lupus panniculitis or lupus profound is a rare variant of chronic photosensitization. Presented for four years, painful lesions on her cutaneous lupus, it can be isolated, or associated with a chronic face, arms, buttocks, consulting, one year later, those lesions have erythematous or systemic lupus, the diagnosis requires a clinical, regressed with facial deformities, weight loss. Dermatological biological and histological correlation. This entity is difficult to treat examination noted several Subcutaneous nodules of different sizes and oral corticoids seem to be the mainstay therapy. We report a with erythematous and pigmented surfaces sitting in the proximal case of a woman who presented systemic lupus erythematous with a part of the arms and limbs, the lower back and buttocks.