Ciliopathies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
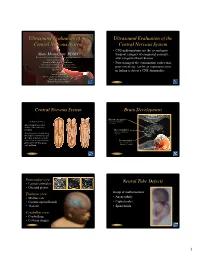
Ultrasound Evaluation of the Central Nervous System
Ultrasound Evaluation of the Ultrasound Evaluation of the Central Nervous System Central Nervous System ••CNSCNS malformations are the second most Mani Montazemi, RDMS frequent category of congenital anomaly, Director of Ultrasound Education & Quality Assurancee after congenital heart disease Baylor College of Medicine Division of Maternal-Fetal Medicine ••PoorPoor timing of the examination, rather than Department of Obstetrics and Gynecology Texas Children’s Hospital, Pavilion for Women poor sensitivity, can be an important factor Houston Texas & in failing to detect a CNS abnormality Clinical Instructor Thomas Jefferson University Hospital Radiology Department Fetal Head Philadelphia, Pennsylvania Fetal Head Central Nervous System Brain Development 9 -13 weeks Rhombencephalon 5th Menstrual Week •Gives rise to hindbrain •4th ventricle Arises from the posterior surface of the embryonic ectoderm Mesencephalon •Gives rise to midbrain A small groove is found along •Aqueduct the midline of the embryo and the edges of this groove fold over to form a neuro tube that Prosencephalon gives rise to the fetal spinal •Gives rise to forebrain rd cord and brain •Lateral & 3 ventricles Fetal Head Fetal Head Ventricular view Neural Tube Defects ••LateralLateral ventricles ••ChoroidChoroid plexus Group of malformations: Thalamic view • Anencephaly ••MidlineMidline falx •Anencephaly ••CavumCavum septiseptipellucidi pellucidi ••CephalocelesCephaloceles ••ThalamiThalami ••SpinaSpina bifida Cerebellar view ••CerebellumCerebellum ••CisternaCisterna magna Fetal -

Educational Paper Ciliopathies
Eur J Pediatr (2012) 171:1285–1300 DOI 10.1007/s00431-011-1553-z REVIEW Educational paper Ciliopathies Carsten Bergmann Received: 11 June 2011 /Accepted: 3 August 2011 /Published online: 7 September 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cilia are antenna-like organelles found on the (NPHP) . Ivemark syndrome . Meckel syndrome (MKS) . surface of most cells. They transduce molecular signals Joubert syndrome (JBTS) . Bardet–Biedl syndrome (BBS) . and facilitate interactions between cells and their Alstrom syndrome . Short-rib polydactyly syndromes . environment. Ciliary dysfunction has been shown to Jeune syndrome (ATD) . Ellis-van Crefeld syndrome (EVC) . underlie a broad range of overlapping, clinically and Sensenbrenner syndrome . Primary ciliary dyskinesia genetically heterogeneous phenotypes, collectively (Kartagener syndrome) . von Hippel-Lindau (VHL) . termed ciliopathies. Literally, all organs can be affected. Tuberous sclerosis (TSC) . Oligogenic inheritance . Modifier. Frequent cilia-related manifestations are (poly)cystic Mutational load kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous Introduction system, occurring either isolated or as part of syn- dromes. Characterization of ciliopathies and the decisive Defective cellular organelles such as mitochondria, perox- role of primary cilia in signal transduction and cell isomes, and lysosomes are well-known -

Ciliopathiesneuromuscularciliopathies Disorders Disorders Ciliopathiesciliopathies
NeuromuscularCiliopathiesNeuromuscularCiliopathies Disorders Disorders CiliopathiesCiliopathies AboutAbout EGL EGL Genet Geneticsics EGLEGL Genetics Genetics specializes specializes in ingenetic genetic diagnostic diagnostic testing, testing, with with ne nearlyarly 50 50 years years of of clinical clinical experience experience and and board-certified board-certified labor laboratoryatory directorsdirectors and and genetic genetic counselors counselors reporting reporting out out cases. cases. EGL EGL Genet Geneticsics offers offers a combineda combined 1000 1000 molecular molecular genetics, genetics, biochemical biochemical genetics,genetics, and and cytogenetics cytogenetics tests tests under under one one roof roof and and custom custom test testinging for for all all medically medically relevant relevant genes, genes, for for domestic domestic andand international international clients. clients. EquallyEqually important important to to improving improving patient patient care care through through quality quality genetic genetic testing testing is is the the contribution contribution EGL EGL Genetics Genetics makes makes back back to to thethe scientific scientific and and medical medical communities. communities. EGL EGL Genetics Genetics is is one one of of only only a afew few clinical clinical diagnostic diagnostic laboratories laboratories to to openly openly share share data data withwith the the NCBI NCBI freely freely available available public public database database ClinVar ClinVar (>35,000 (>35,000 variants variants on on >1700 >1700 genes) genes) and and is isalso also the the only only laboratory laboratory with with a a frefree oen olinnlein dea dtabtaabsaes (eE m(EVmCVlaCslas)s,s f)e, afetuatruinrgin ag vaa vraiarniatn ctl acslasisfiscifiactiaotino sne saercahrc ahn adn rde rpeoprot rrte rqeuqeuset sint tinetrefarcfaec, ew, hwichhic fha cfailcitialiteatse rsa praidp id interactiveinteractive curation curation and and reporting reporting of of variants. -

Pfeiffer Syndrome Type II Discovered Perinatally
Diagnostic and Interventional Imaging (2012) 93, 785—789 CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector LETTER / Musculoskeletal imaging Pfeiffer syndrome type II discovered perinatally: Report of an observation and review of the literature a,∗ a a a H. Ben Hamouda , Y. Tlili , S. Ghanmi , H. Soua , b c b a S. Jerbi , M.M. Souissi , H. Hamza , M.T. Sfar a Unité de néonatologie, service de pédiatrie, CHU Tahar Sfar, 5111 Mahdia, Tunisia b Service de radiologie, CHU Tahar Sfar, 5111 Mahdia, Tunisia c Service de gynéco-obstétrique, CHU Tahar Sfar, 5111 Mahdia, Tunisia Pfeiffer syndrome, described for the first time by Pfeiffer in 1964, is a rare hereditary KEYWORDS condition combining osteochondrodysplasia with craniosynostosis [1]. This syndrome is Pfeiffer syndrome; also called acrocephalosyndactyly type 5, which is divided into three sub-types. Type I Cloverleaf skull; is the classic Pfeiffer syndrome, with autosomal dominant transmission, often associated Craniosynostosis; with normal intelligence. Types II and III occur as sporadic cases in individuals who have Syndactyly; craniosynostosis with broad thumbs, broad big toes, ankylosis of the elbows and visceral Prenatal diagnosis abnormalities [2]. We report a case of Pfeiffer syndrome type II, discovered perinatally, which is distinguished from type III by the skull appearing like a cloverleaf, and we shall discuss the clinical, radiological and evolutive features and the advantage of prenatal diagnosis of this syndrome with a review of the literature. Observation The case involved a male premature baby born at 36 weeks of amenorrhoea with multiple deformities at birth. The parents were not blood-related and in good health who had two other boys and a girl with normal morphology. -

Unraveling the Genetics of Joubert and Meckel-Gruber Syndromes
Journal of Pediatric Genetics 3 (2014) 65–78 65 DOI 10.3233/PGE-14090 IOS Press Unraveling the genetics of Joubert and Meckel-Gruber syndromes Katarzyna Szymanska, Verity L. Hartill and Colin A. Johnson∗ Department of Ophthalmology and Neuroscience, University of Leeds, Leeds, UK Received 27 May 2014 Revised 11 July 2014 Accepted 14 July 2014 Abstract. Joubert syndrome (JBTS) and Meckel-Gruber syndrome (MKS) are recessive neurodevelopmental conditions caused by mutations in proteins that are structural or functional components of the primary cilium. In this review, we provide an overview of their clinical diagnosis, management and molecular genetics. Both have variable phenotypes, extreme genetic heterogeneity, and display allelism both with each other and other ciliopathies. Recent advances in genetic technology have significantly improved diagnosis and clinical management of ciliopathy patients, with the delineation of some general genotype-phenotype correlations. We highlight those that are most relevant for clinical practice, including the correlation between TMEM67 mutations and the JBTS variant phenotype of COACH syndrome. The subcellular localization of the known MKS and JBTS proteins is now well-described, and we discuss some of the contemporary ideas about ciliopathy disease pathogenesis. Most JBTS and MKS proteins localize to a discrete ciliary compartment called the transition zone, and act as structural components of the so-called “ciliary gate” to regulate the ciliary trafficking of cargo proteins or lipids. Cargo proteins include enzymes and transmembrane proteins that mediate intracellular signaling. The disruption of transition zone function may contribute to the ciliopathy phenotype by altering the composition of the ciliary membrane or axoneme, with impacts on essential developmental signaling including the Wnt and Shh pathways as well as the regulation of secondary messengers such as inositol-1,4,5-trisphosphate (InsP3) and cyclic adenosine monophosphate (cAMP). -

A 10-Year-Old Girl with Joubert Syndrome and Chronic Kidney Disease and Its Related Complications
4226 Letter to the Editor A 10-year-old girl with Joubert syndrome and chronic kidney disease and its related complications Chong Tian1#, Jiaxiang Chen1,2#, Xing Ming1, Xianchun Zeng1, Rongpin Wang1 1Department of Medical Imaging, Guizhou Provincial People’s Hospital, Guiyang, China; 2Guizhou University School of Medicine, Guiyang, China #These authors contributed equally to this work. Correspondence to: Rongpin Wang. Department of Medical Imaging, Guizhou Provincial People’s Hospital, Zhongshan East Road 83, Guiyang 550002, China. Email: [email protected]. Submitted Aug 05, 2020. Accepted for publication Apr 01, 2021. doi: 10.21037/qims-20-943 View this article at: http://dx.doi.org/10.21037/qims-20-943 Introduction and ankles was tolerated without redness, swelling, fistula, or sinus tract surrounding the skin. She had been healthy in Joubert syndrome (JS) is a rare genetic disorder of recessive the past, and her academic performance was moderate. She neurodevelopmental disorder characterized by distinctive was a picky eater and had a mild short stature but showed cerebellar vermis and mid-hindbrain hypoplasia/dysplasia no other development dysplasia. Nystagmus, oculomotor called the “molar tooth sign” (MTS) (1). Patients present apraxia, hypotonia, and abnormal breathing patterns were with symptoms characteristic of hypotonia in infancy not observed. Concerning the patient’s family history, her and later develop ataxia, ocular motor apraxia, and may mother and father appeared normal, but their first child died also present with developmental delays or intellectual retardation. Defined by the central nervous system features, of renal failure at the age of 4, their second-born twin sons JS also affects many other organs, such as the kidneys, liver, died in the perinatal period (causes unknown), and a third and bones. -

Molar Tooth Sign of the Midbrain-Hindbrain Junction
American Journal of Medical Genetics 125A:125–134 (2004) Molar Tooth Sign of the Midbrain–Hindbrain Junction: Occurrence in Multiple Distinct Syndromes Joseph G. Gleeson,1* Lesley C. Keeler,1 Melissa A. Parisi,2 Sarah E. Marsh,1 Phillip F. Chance,2 Ian A. Glass,2 John M. Graham Jr,3 Bernard L. Maria,4 A. James Barkovich,5 and William B. Dobyns6** 1Division of Pediatric Neurology, Department of Neurosciences, University of California, San Diego, California 2Division of Genetics and Development, Children’s Hospital and Regional Medical Center, University of Washington, Washington 3Medical Genetics Birth Defects Center, Ahmanson Department of Pediatrics, Cedars-Sinai Medical Center, UCLA School of Medicine, Los Angeles, California 4Department of Child Health, University of Missouri, Missouri 5Departments of Radiology, Pediatrics, Neurology, Neurosurgery, University of California, San Francisco, California 6Department of Human Genetics, University of Chicago, Illinois The Molar Tooth Sign (MTS) is defined by patients with these variants of the MTS will an abnormally deep interpeduncular fossa; be essential for localization and identifica- elongated, thick, and mal-oriented superior tion of mutant genes. ß 2003 Wiley-Liss, Inc. cerebellar peduncles; and absent or hypo- plastic cerebellar vermis that together give KEY WORDS: Joubert; molar tooth; Va´ r- the appearance of a ‘‘molar tooth’’ on axial adi–Papp; OFD-VI; COACH; brain MRI through the junction of the mid- Senior–Lo¨ ken; Dekaban– brain and hindbrain (isthmus region). It was Arima; cerebellar vermis; first described in Joubert syndrome (JS) hypotonia; ataxia; oculomo- where it is present in the vast majority of tor apraxia; kidney cysts; patients with this diagnosis. -

A Anencephaly
Glossary of Birth Anomaly Terms: A Anencephaly: A deadly birth anomaly where most of the brain and skull did not form. Anomaly: Any part of the body or chromosomes that has an unusual or irregular structure. Aortic valve stenosis: The aortic valve controls the flow of blood from the left ventricle of the heart to the aorta, which takes the blood to the rest of the body. If there is stenosis of this valve, the valve has space for blood to flow through, but it is too narrow. Atresia: Lack of an opening where there should be one. Atrial septal defect: An opening in the wall (septum) that separates the left and right top chambers (atria) of the heart. A hole can vary in size and may close on its own or may require surgery. Atrioventricular septal defect (endocardial cushion defect): A defect in both the lower portion of the atrial septum and the upper portion of the ventricular septum. Together, these defects make a large opening (canal) in the middle part of the heart. Aniridia (an-i-rid-e-a): An eye anomaly where the colored part of the eye (called the iris) is partly or totally missing. It usually affects both eyes. Other parts of the eye can also be formed incorrectly. The effects on children’s ability to see can range from mild problems to blindness. To learn more about aniridia, go to the U.S. National Library of Medicine website. Anophthalmia/microphthalmia (an-oph-thal-mia/mi-croph-thal-mia): Birth anomalies of the eyes. In anophthalmia, a baby is born without one or both eyes. -

GENETIC STUDY of RENAL DISEASES (Nephroref Global®) by MASSIVE SEQUENCING (NGS)
Pablo Iglesias, 57 – Polígono Gran Via Sur 08908 L'Hospitalet de Llobregat (Barcelona) Tel. 932 593 700 – Fax. 932 845 000 GENETIC STUDY OF RENAL DISEASES (NephroRef Global®) BY MASSIVE SEQUENCING (NGS) Request No.: 000 Client: - Analysis code: 55580 Patient Name: xxx Date of Birth: N/A Patient Ref.: xxx Gender: Female Sample Type: Blood EDTA Sample Arrival Date: DD/MM/AAAA Date of Result: DD/MM/AAAA Clinical information: A 9-year-old patient with a nephrotic syndrome without response to corticosteroid therapy. She has nephrotic-range proteinuria with microhematuria, hypoalbuminemia with hypercholesterolemia and normal glomerular filtration. Paternal aunt with cortico-resistant nephrotic syndrome with evolution to end-stage renal failure that required renal transplantation at age 13. RESULT AND INTERPRETATION The presence of a heterozygous likely pathogenic variant has been identified. In addition, the presence of a heterozygous variant of uncertain clinical significance (VUS) has been identified.(See Interpretation and recommendations) The complete list of studied genes is available in Annex 1. (Methodology) The list of reported genes and coverage details is available in Table 1. (Methodology) Gene Variant* Zygosity Inheritance pattern Classification^ NPHS2 NM_014625.3: c.842A>C Heterozygosis Autosomal Recessive Likely Patogénica p.(Glu281Ala) INF2 NM_022489.3: c.67T>A Heterozygosis Autosomal Recessive VUS p.(Ser23Thr) * Nomenclature according to HGVS v15.11 ^ Based on the recommendations of the American College of Medical Genetics and Genomics (ACMG) Physician, technical specialist responsible for Clinical Analysis: Jaime Torrents Pont. The results relate to samples received and analysed. This report may not be reproduced in part without permission. This document is addressed to the addressee and contains confidential information. -

SDCCAG8 Interacts with RAB Effector Proteins RABEP2 and ERC1 and Is Required for Hedgehog Signaling
SDCCAG8 Interacts with RAB Effector Proteins RABEP2 and ERC1 and Is Required for Hedgehog Signaling Airik, Rannar; Schueler, Markus; Airik, Merlin; Cho, Jang; Ulanowicz, Kelsey A; Porath, Jonathan D; Hurd, Toby W; Bekker-Jensen, Simon; Schrøder, Jacob Morville; Andersen, Jens S.; Hildebrandt, Friedhelm Published in: P L o S One DOI: 10.1371/journal.pone.0156081 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Airik, R., Schueler, M., Airik, M., Cho, J., Ulanowicz, K. A., Porath, J. D., Hurd, T. W., Bekker-Jensen, S., Schrøder, J. M., Andersen, J. S., & Hildebrandt, F. (2016). SDCCAG8 Interacts with RAB Effector Proteins RABEP2 and ERC1 and Is Required for Hedgehog Signaling. P L o S One, 11(5), [e0156081]. https://doi.org/10.1371/journal.pone.0156081 Download date: 04. Oct. 2021 RESEARCH ARTICLE SDCCAG8 Interacts with RAB Effector Proteins RABEP2 and ERC1 and Is Required for Hedgehog Signaling Rannar Airik1¤‡*, Markus Schueler1, Merlin Airik1, Jang Cho1, Kelsey A. Ulanowicz2, Jonathan D. Porath1, Toby W. Hurd3, Simon Bekker-Jensen4, Jacob M. Schrøder5, Jens S. Andersen5, Friedhelm Hildebrandt1,6‡* 1 Department of Medicine, Division of Nephrology, Boston Children’s Hospital, Boston, Massachusetts, United States of America, 2 Department of Pediatrics, Division of Nephrology, Children’s Hospital of a11111 Pittsburgh of UPMC, Pittsburgh, Pennsylvania, United States of America, 3 Medical Research Council Human Genetics Unit, Institute of -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Ciliopathies Gene Panel
Ciliopathies Gene Panel Contact details Introduction Regional Genetics Service The ciliopathies are a heterogeneous group of conditions with considerable phenotypic overlap. Levels 4-6, Barclay House These inherited diseases are caused by defects in cilia; hair-like projections present on most 37 Queen Square cells, with roles in key human developmental processes via their motility and signalling functions. Ciliopathies are often lethal and multiple organ systems are affected. Ciliopathies are London, WC1N 3BH united in being genetically heterogeneous conditions and the different subtypes can share T +44 (0) 20 7762 6888 many clinical features, predominantly cystic kidney disease, but also retinal, respiratory, F +44 (0) 20 7813 8578 skeletal, hepatic and neurological defects in addition to metabolic defects, laterality defects and polydactyly. Their clinical variability can make ciliopathies hard to recognise, reflecting the ubiquity of cilia. Gene panels currently offer the best solution to tackling analysis of genetically Samples required heterogeneous conditions such as the ciliopathies. Ciliopathies affect approximately 1:2,000 5ml venous blood in plastic EDTA births. bottles (>1ml from neonates) Ciliopathies are generally inherited in an autosomal recessive manner, with some autosomal Prenatal testing must be arranged dominant and X-linked exceptions. in advance, through a Clinical Genetics department if possible. Referrals Amniotic fluid or CV samples Patients presenting with a ciliopathy; due to the phenotypic variability this could be a diverse set should be sent to Cytogenetics for of features. For guidance contact the laboratory or Dr Hannah Mitchison dissecting and culturing, with ([email protected]) / Prof Phil Beales ([email protected]) instructions to forward the sample to the Regional Molecular Genetics Referrals will be accepted from clinical geneticists and consultants in nephrology, metabolic, laboratory for analysis respiratory and retinal diseases.