DC Source Water Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5
NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5 DATABASE DESIGN DOCUMENTATION AND DATA DICTIONARY 1 June 2013 Prepared for: United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, Maryland 21403 Prepared By: Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 Prepared for United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, MD 21403 By Jacqueline Johnson Interstate Commission on the Potomac River Basin To receive additional copies of the report please call or write: The Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 301-984-1908 Funds to support the document The Non-Tidal Benthic Monitoring Database: Version 3.0; Database Design Documentation And Data Dictionary was supported by the US Environmental Protection Agency Grant CB- CBxxxxxxxxxx-x Disclaimer The opinion expressed are those of the authors and should not be construed as representing the U.S. Government, the US Environmental Protection Agency, the several states or the signatories or Commissioners to the Interstate Commission on the Potomac River Basin: Maryland, Pennsylvania, Virginia, West Virginia or the District of Columbia. ii The Non-Tidal Benthic Monitoring Database: Version 3.5 TABLE OF CONTENTS BACKGROUND ................................................................................................................................................. 3 INTRODUCTION .............................................................................................................................................. -

Bridging the Union. Arch
? .,,~' ml i " | j z- •" VOl~!:~i~:Nb. 11 SERVING THE PEOPLE OF CABIN JOHN AND BEYOND AUGUST 1975 i j ..... - FUTURE OF GLEN ECHO PARK UNCERTAIN By Shelly Keller Glen Echo- Park needs some help to realize its amazing potentiM as a national, cultural resource center, especially now that the Office of Manageirient and Bud- get seems serious about giving the Park to the Maryland National Capital:Park and Planning Commission or some other State or County agency. As of now, the Park is a "special pro- gram" of the NationalPark Service with the land title residing with GSA. The ,~ Park was to come.under the NPS admini-:~ii" ~. " strative jurisdiction through'the land transfer, but because OMB has not yet moved to finalize the transfer of the land title, there is no legislative description for ~ the Park• : - ~" Many people within the Park Service and especially people involved in the Park itself, fear that OMB might decide that: Glen Echo Park is too experimental, too .:,'. urban and too unlike other National Parks to be ~y_en_ to NPS. Some NPS people reeF f eh-T~-6:~ay; others, andmembers of Congress seem to support efforts to U.S. Con#essman Gilbert Gude and Administrative Aide Keith Schiszik at the July 24th •~ create more urban park situations. meeting abou~ .the .future of Union Arch Bridge. Pho'to by Linda Ford "" In a~i~'~er'~o-'Goti~essmanGude on ~ July 22, Paul O'Neill, Deputy Director of OMB, stated, "I don't think we're far a- BRIDGING THE UNION. ARCH GAP part regarding the future use ofthis pro- by Cat Feild perty and the undesirability of commer- cial development of this site• The issue Believe it or not, the Union Arch Bridge is not a dormant issue. -

Washington Suburban Sanitary Commission – Video Streaming and Archiving Meetings and Late Payment Charges (MC/PG 100-21) (Charkoudian)
DocuSign Envelope ID: 8DD43C77-6A9A-477D-A5DC-5CAFBC8C4614 COMMISSION SUMMARY AGENDA CATEGORY: Intergovernmental Relations Office ITEM NUMBER: 1 DATE: April 21, 2021 SUBJECT Legislative Update SUMMARY Updates on WSSC Water-related bills for the 2021 Legislative Session. The legislative update is current as of April 5, 2021, and will be updated SPECIAL COMMENTS as legislation is received and/or positions are taken. CONTRACT NO./ N/A REFERENCE NO. N/A COSTS AMENDMENT/ CHANGE ORDER NO. N/A AMOUNT MBE PARTICIPATION N/A PRIOR STAFF/ COMMITTEE REVIEW Carla A. Reid, General Manager/CEO Monica Johnson, Deputy General Manager, Strategy & Partnerships PRIOR STAFF/ Karyn A. Riley, Director, Intergovernmental Relations Office COMMITTEE APPROVALS RECOMMENDATION TO COMMISSION COMMISSION ACTION DocuSign Envelope ID: 8DD43C77-6A9A-477D-A5DC-5CAFBC8C4614 SESSION 2021 LEGISLATIVE UPDATE As of April 5, 2021 WSSC Water Sponsored Legislation Bill # Title (Sponsor)/Description Position Status/Notes Washington Suburban Sanitary Hearings: Commission – Board of Ethics - Financial Disclosure Statements - Late Senate Education, Health and Fees (MC/PG 102-21) Environmental Affairs 4/6/2021 Authorizes the WSSC Board of Ethics to impose a late fee on individuals who file House – Passed (136-0) late or fail to file required financial HB 501 SUPPORT 3/18/2021 (MC/PG 102-21) disclosure statements. (10/21/2020) Positions: PGHD – FAV (1/22/21) MCHD – FAV (1/29/21) MCCE – Support MCCC – Support PGCC – Support WSSC Water Related Legislation Bill # Title (Sponsor)/Description Position Status/Notes Renewable Energy Portfolio Standard – Wastewater, Thermal, and Other Renewable Sources (D.E. Davis) As amended, expands Tier 1 renewable Hearings: energy sources to include raw or treated wastewater used as a heat source or heat Senate Finance SUPPORT HB 561 sink for a heating or cooling system, 3/30/2021 (1/27/2021) subject to specified requirements. -

Brook Trout Outcome Management Strategy
Brook Trout Outcome Management Strategy Introduction Brook Trout symbolize healthy waters because they rely on clean, cold stream habitat and are sensitive to rising stream temperatures, thereby serving as an aquatic version of a “canary in a coal mine”. Brook Trout are also highly prized by recreational anglers and have been designated as the state fish in many eastern states. They are an essential part of the headwater stream ecosystem, an important part of the upper watershed’s natural heritage and a valuable recreational resource. Land trusts in West Virginia, New York and Virginia have found that the possibility of restoring Brook Trout to local streams can act as a motivator for private landowners to take conservation actions, whether it is installing a fence that will exclude livestock from a waterway or putting their land under a conservation easement. The decline of Brook Trout serves as a warning about the health of local waterways and the lands draining to them. More than a century of declining Brook Trout populations has led to lost economic revenue and recreational fishing opportunities in the Bay’s headwaters. Chesapeake Bay Management Strategy: Brook Trout March 16, 2015 - DRAFT I. Goal, Outcome and Baseline This management strategy identifies approaches for achieving the following goal and outcome: Vital Habitats Goal: Restore, enhance and protect a network of land and water habitats to support fish and wildlife, and to afford other public benefits, including water quality, recreational uses and scenic value across the watershed. Brook Trout Outcome: Restore and sustain naturally reproducing Brook Trout populations in Chesapeake Bay headwater streams, with an eight percent increase in occupied habitat by 2025. -

Gazetteer of West Virginia
Bulletin No. 233 Series F, Geography, 41 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIKECTOU A GAZETTEER OF WEST VIRGINIA I-IEISTRY G-AN3STETT WASHINGTON GOVERNMENT PRINTING OFFICE 1904 A» cl O a 3. LETTER OF TRANSMITTAL. DEPARTMENT OP THE INTEKIOR, UNITED STATES GEOLOGICAL SURVEY, Washington, D. C. , March 9, 190Jh SIR: I have the honor to transmit herewith, for publication as a bulletin, a gazetteer of West Virginia! Very respectfully, HENRY GANNETT, Geogwvpher. Hon. CHARLES D. WALCOTT, Director United States Geological Survey. 3 A GAZETTEER OF WEST VIRGINIA. HENRY GANNETT. DESCRIPTION OF THE STATE. The State of West Virginia was cut off from Virginia during the civil war and was admitted to the Union on June 19, 1863. As orig inally constituted it consisted of 48 counties; subsequently, in 1866, it was enlarged by the addition -of two counties, Berkeley and Jeffer son, which were also detached from Virginia. The boundaries of the State are in the highest degree irregular. Starting at Potomac River at Harpers Ferry,' the line follows the south bank of the Potomac to the Fairfax Stone, which was set to mark the headwaters of the North Branch of Potomac River; from this stone the line runs due north to Mason and Dixon's line, i. e., the southern boundary of Pennsylvania; thence it follows this line west to the southwest corner of that State, in approximate latitude 39° 43i' and longitude 80° 31', and from that corner north along the western boundary of Pennsylvania until the line intersects Ohio River; from this point the boundary runs southwest down the Ohio, on the northwestern bank, to the mouth of Big Sandy River. -

TROUT Stocking – Lakes and Ponds Code No
TROUT Stocking – Lakes and Ponds Code No. Stockings .......Period Code No. Stockings .......Period Code No. Stockings .......Period Q One ...........................1st week of March Twice a month .............. February-April CR Varies ...........................................Varies BW One ........................................... January M One each month ........... February-May One .................................................. May W Two..........................................February MJ One each month ............January-April One ........................................... January One each week ....................March-May Y One ................................................. April BA One each week ...................................... X After April 1 or area is open to public One ...............................................March F weeks of October 19 and 26 Lake or Pond ‒ County Code Lake or Pond ‒ County Code Anawalt – McDowell M Laurel – Mingo MJ Anderson – Kanawha BA Lick Creek – Wayne MJ Baker – Ohio Q Little Beaver – Raleigh MJ Barboursville – Cabell BA Logan County Airport – Logan Q Bear Rock Lakes – Ohio BW Mason Lake – Monongalia M Berwind – McDowell M Middle Wheeling Creek – Ohio BW Big Run – Marion Y Miletree – Roane BA Boley – Fayette M Mill Creek – Barbour M Brandywine – Pendleton BW-F Millers Fork – Wayne Q Brushy Fork – Pendleton BW Mountwood – Wood MJ Buffalo Fork – Pocahontas BW-F Newburg – Preston M Cacapon – Morgan W-F New Creek Dam 14 – Grant BW-F Castleman Run – Brooke, Ohio BW Pendleton – Tucker -
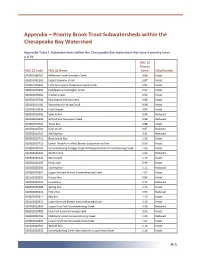
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

Nitrate Tmdl Development: the Muddy Creek/Dry River Case Study
NITRATE TMDL DEVELOPMENT: THE MUDDY CREEK/DRY RIVER CASE STUDY Teresa B. Culver Kathryn A. Neeley Shaw L. Yu Harry X. Zhang, Andrew L. Potts Troy R. Naperala Civil Engineering Department University of Virginia In 1972, Section 303(d) of the Clean Water Act (CWA) estuaries, which includes approximately 475,000 established the regulatory concept of a Total Maximum kilometers (300,000 miles) of river and shoreline Daily Load (TMDL) as the maximum loading rate of a (USEPA, 2001). The USEPA suggests that states plan pollutant that a receiving water can assimilate without to complete the TMDL’s with a maximum planning resultant water quality impairments with respect to the time frame of 13 years (Perciasepe, 1997). Given that applicable water quality standards. The CWA specified there are typically hundreds of impaired waterbodies per that the watershed-level TMDL approach should be state, the effort required to meet these timelines is used to systematically manage both point and non-point enormous. Furthermore, in many states, court orders or source pollution. However, it was not until the 1990’s consent decrees now specify the rate at which TMDL’s after a series of legal actions that the TMDL program must be established (USEPA, 2001). In addition, active has been actively pursued at federal and state levels. and effective community involvement is expected in a The TMDL concept has now grown into a TMDL project, so modeling analysis should be made comprehensive surface water management approach. A intelligible to community members. thorough description and guidance for the TMDL program can be found at the U.S. -

An Analysis of Clean Water Act Compliance, July 2003- December 2004
March 2006 An analysis of Clean Water Act compliance, July 2003- December 2004 Troubled Waters An analysis of Clean Water Act compliance, July 2003- December 2004 March 2006 Troubled Waters i Acknowledgments Written by Christy Leavitt, Clean Water Advocate with MaryPIRG Foundation. © 2006, MaryPIRG Foundation Cover photo: Photodisc. The author would like to thank Alison Cassady, Research Director with MaryPIRG Foundation, for her editorial assistance and contributions to this report. Additional thanks to the numerous staff at state environmental protection agencies across the country for reviewing the data for accuracy. The recommendations are those of the MaryPIRG Foundation and do not necessarily reflect the views of our funders. To obtain additional copies of this report, visit our website or send a check for $25 made payable to MaryPIRG Foundation at the following address: MaryPIRG Foundation 3121 St. Paul St., Suite 26 Baltimore, MD 21218 (410) 467-0439 www.marypirg.org Troubled Waters ii Table of Contents Executive Summary 1 Introduction: The State of America’s Waters 3 Background: A Permit to Pollute 4 Findings: America’s Troubled Waterways 7 The Bush Administration’s Assault on the Clean Water Act 14 Recommendations 17 Methodology 20 Appendix A. Facilities Exceeding Their Clean Water Act Permits for at Least 9 of the 18 Reporting Periods between July 1, 2003 and December 31, 2004 22 Appendix B. All Maryland Facilities Exceeding their Clean Water Act Permits at Least Once between July 1, 2003 and December 31, 2004 32 Troubled Waters iii Executive Summary hen drafting the Clean Water Act in 1972, legislators set the goals of making all U.S. -

Studies of Longitudinal Stream Profiles in Virginia and Maryland
Studies of Longitudinal Stream Profiles in Virginia and Maryland By JOHN T. HACK SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL. SURVEY PROFESSIONAL PAPER 294-B Preliminary results of a study of the form of small river valleys in relation to geology. Some factors controlling the longitudinal profiles of streams are described in q'uantitative terms UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1957 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. - Price 75 cents (paper cover) CONTENTS Page Peg* AbstractL 45 Relation of particle size of material on the bed to stream IntroductionL 47 lengthL 68 Methods of study and definitions of factors measuredL 47 Mathematical expression of the longitudinal profile and Description of areas studied L 49 its relation to particle size of material on the bedL 69 Middle River basinL 50 Mathematical expression in previous work on longitudinal North River basinL 50 profilesL 74 Alluvial terrace areasL 50 Origin and composition of stream-bed materialL 74 Calfpasture River basinL 50 Franks Mill reach of the Middle RiverL 76 Tye River basin L 52 Eidson CreekL 81 Gillis FallsL 52 East Dry BranchL 82 Coastal Plain streamsL 53 North RiverL 84 Factors determining the slope of the stream channelL 53 Calfpasture ValleyL 84 Discharge and drainage areaL 54 Gillis FallsL 85 Size of material on the stream bedL 54 Ephemeral streams in areas of residuumL 85 Channel cross sectionL 61 Some factors controlling variations in size: conclusions_ _ _ 86 Summary of factors controlling channel slopeL 61 The longitudinal profile and the cycle of erosionL 87 Factors determining the position of the channel in space: the References cited L 94 shape of the long profileL 63 IndexL 95 Relation of stream length to drainage area L 63 ILLUSTRATIONS Pag e Page PLATE99. -

Testimony of Mr
Testimony of Mr. Thomas P. Jacobus General Manager, Washington Aqueduct Performance Oversight Fiscal Years 2017 and 2018 Before the Committee on Transportation and the Environment Council of the District of Columbia Friday, March 2, 2018 Chairperson Cheh and members of the Committee, thank you for inviting me today to testify on the performance of Washington Aqueduct. I am Tom Jacobus, general manager of Washington Aqueduct. We have the responsibility to provide wholesale water service to DC Water for further distribution to the residents, businesses and other activities in the District of Columbia. Engagement with Wholesale Customers Washington Aqueduct has a highly-effective and valuable relationship with our largest wholesale customer: DC Water. There is daily interaction at the executive, manager and staff levels to address ongoing production operations to ensure DC Water is receiving the quality and quantity of water expected by its customers. Both DC Water and Washington Aqueduct have added individual expertise, engineering, and scientific capability, and we have shown ourselves to be very capable to respond to any unusual or unforeseen event. We share data and process information with DC Water, and we receive from them technical insight and innovative ideas. Washington Aqueduct also serves two wholesale customers in Northern Virginia: Arlington County and Fairfax Water. Our three wholesale customers, acting under the provisions of the Memorandum of Understanding that established a Wholesale Customer Board, fund all operations and our capital improvements. Water Quality and Water Supply Risk Reduction Washington Aqueduct has approached our stewardship of the drinking water resource through the prism of risk reduction. Over ten years ago, the Wholesale Customer Board requested that we evaluate advanced treatment options, such as granular activated carbon adsorption, membrane treatment, ozone/biofiltration, and ultraviolet disinfection. -

GAME FISH STREAMS and RECORDS of FISHES from the POTOMAC-SHENANDOAH RIVER SYSTEM of VIRGINIA
) • GAME FISH STREAMS AND RECORDS OF FISHES FROM THE POTOMAC-SHENANDOAH RIVER SYSTEM Of VIRGINIA Robert D. Ross Associate Professor of Biology Technical Bulletin 140 April 1959 Virginia Agricultural Experiment Station Virginia Polytechnic Institute Blacksburg, Virginia ACKNOWLEDGMENTS The writer is grateful to Eugene S. Surber, Robert G. Martin and Jack M. Hoffman who directed the survey and gave their help and encouragement. A great deal of credit for the success of the Survey is due to all game wardens who rendered invaluable assistance. Special thanks are due to many sportsmen and assistant game wardens who helped the field crew. Personnel of the Commission of Game and Inland Fisheries, who helped in the work from time to time were William Fadley, William Hawley, Max Carpenter and Dixie L. Shumate. The Virginia Academy of Science gener- ously donated funds for the purchase of alcohol in which the fish collection was preserved. GAME FISH STREAMS AND RECORDS OF FISHES FROM THE SHENANDOAH-POTOMAC RIVER SYSTEMS OF VIRGINIA Robert D. Ross Associate Professor of Biology Virginia Polytechnic Institute INTRODUCTION From June 15 to September 15, 1956, the Commission of Game and Inland Fisheries, Division of Fisheries, Richmond, Virginia, undertook a survey of a major part of the Shenandoah-Potomac River watershed in Virginia. This work was done as Federal Aid Project No. F-8-R-3, in cooperation with Vir- ginia Cooperative Wildlife Research Unit, under the direction of Robert G. Martin, Dingell-Johnson Coordinator, and Jack M. Hoffman, Leader. Robert D. Ross, Crew Leader, and David W. Robinson and Charles H. Hanson worked in the field.