DRIVING HEALTH INNOVATION Research Report 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiation Medicine Program
Radiation Medicine Program ANNUAL 2020 REPORT 2021 VALUES MISSION Innovation Advance exemplary radiation Excellence medicine through patient Collaboration care, research & education Accountability in partnership with CONTENTS Integrity our patients & community 4 A Message from the Chief 6 Program Overview 7 2020: The Year in Numbers 8 Strategic Roadmap to 2026 VISION 10 Clinical Care CURE EVOLVE 15 Quality & Safety Predictive Health & Precision Radiation Medicine. Advanced Particle Adaptive Radiotherapy Therapy & Theranostics 18 Education Personalized Care. 21 Research Global Impact. 26 Team RMP COMFORT & CONNECT Systems to Maximize CONFIDENCE Innovation & Wellbeing Technology-enabled Patient Experience Transformation A MESSAGE FROM THE CHIEF The Radiation Medicine Program (RMP) at the Princess Margaret Cancer Centre is committed to delivering the highest standard of patient care. Over the RMP’s innovative education programs continue to thrive and attract a diverse group of national and international attendees. Our award-winning Accelerated past year, our dynamic multidisciplinary team of radiation oncologists, medical physicists, radiation therapists, administrators, and support staff have worked Education Program (AEP) demonstrated extraordinary resourcefulness this past year, standing strong amidst the pandemic, and continuing to provide top- together to advance our vision of “Precision Radiation Medicine. Personalized Care. Global Impact.” RMP continues to uphold our foundational values of level education to a broad spectrum of learners. -

Canada Scientific Advisory Committee (CSAC)
Canada Scientific Advisory Committee (CSAC) Alan Bernstein, PhD – Co-chairperson Canadian Institute for Advanced Research Toronto, Ontario Alan Bernstein is President of the Canadian Institute for Advanced Research (CIFAR), Canada’s global research institute. From 2008- 2011, Bernstein was the executive director of the Global HIV Vaccine Enterprise, an international alliance of researchers and funders charged with accelerating the search for an HIV vaccine. Previously, he served as the founding president of the Canadian Institutes of Health Research (2000-2007), Canada’s federal agency for the support of health research. In that capacity, he led the transformation of health research in Canada. After receiving his PhD from the University of Toronto, and following postdoctoral work in London, Bernstein joined the Ontario Cancer Institute (1974-1985). In 1985, he joined the new Samuel Lunenfeld Research Institute in Toronto, was named Associate Director in 1988 and then Director of Research (1994-2000). Internationally known for his contributions to our understanding of the molecular basis of cancer, Bernstein has made extensive contributions to the study of stem cells, hematopoiesis and cancer. He chairs or is a member of advisory and review boards in Canada, the US, UK and Italy. Bernstein has received numerous awards and honourary degrees for his contributions to science, including the 2008 Gairdner Wightman Award, induction into the Canadian Medical Hall of Fame, and the Henry G. Friesen International Prize in Health Research. He is a Senior Research Fellow of Massey College, received the Order of Ontario in 2018 and was appointed an Officer of the Order of Canada in 2002. -

ANNUAL REPORT 2015/2016 Message from the Minister of Research, Innovation and Science
ANNUAL REPORT 2015/2016 Message from the Minister of Research, Innovation and Science On behalf of the Government of Ontario I am pleased to have the opportunity to extend my thanks to the Ontario Institute for Cancer Research (OICR) for another successful year in its progress to meet the cancer challenge. A report issued recently by Cancer Care Ontario tells us that one in two Ontarians will develop cancer in their lifetime and cancer is the leading cause of death in the province. We have to do everything possible to alleviate the burden of cancer on Ontario families by improving the prevention, detection, diagnosis and treatment of cancer. We are proud of our investment in OICR over the last 10 years and recently approved the Institute’s Strategic Plan for 2016-2021. OICR has demonstrated leadership in the cancer community not only in its research programs but also in its focus on moving discoveries into the clinic. An example is the development of a novel oncolytic viral immunotherapy which is now in clinical trials. Through OICR’s commercialization partner, the Fight Against Cancer Innovation Trust, financial and in-kind support are attracted which makes it possible to accelerate development and therefore bring new hope to patients. The past year has been one of accomplishment and one of transition. Dr. Calvin Stiller, who was instrumental in the creation of the Institute, has stepped down as Chair of the Board of Directors. We are most grateful for his vision and leadership. Dr. Tom Hudson, who built the Institute, attracted talented researchers and launched provincial, national and international initiatives that are helping to make Ontario a world leader in cancer research, has moved on to the next phase of his career. -

Princess Margaret Cancer Centre Annual Report
2 015 A NNU A L REPORT 3 Leadership Message 4 Our Program 4 Who we are Welcome to the 2015 Annual Report for the Princess Margaret Cancer Centre at the University Health Network (UHN). This year proved to be both exciting and engaging as we continue to deliver revolutionary cancer care for our patients, and we are looking 6 Clinical Care forward to sharing the progress we have made. The 2015 edition showcases the recent 6 Our Clinical Programs LEADERSHIP activities and accomplishments of our people, departments, disease groups, and research and education programs. As one of the largest comprehensive cancer treatment facilities in the world, we have a great deal to share as we continue our efforts to be on the 8 Personalized Cancer Medicine MESSAGE frontiers of medical, surgical and radiation oncology, embracing the latest technology 8 Our Strategy and international best-practices, and setting standards for patient care. 9 We Are Transforming Patient Care In 2015, we celebrated 20 years of our presence on University Avenue; the hub of the discovery district. The move not only signified our commitment to meet the increased 11 We Are Augmenting Correlative Cancer Biology demands and evolving needs of our patients, but also encouraged collaboration, innovation, and research, enabling us to continue making progress in conquering cancer. 12 We Are Accelerating Guided Therapeutics Today, we again embrace change as we drive implementation of our space plan, with Marnie Escaf MHA, HBBA 15 We Are Expanding Novel Therapeutics Senior Vice President a focus on redeveloping facilities to improve the patient experience, including patient Executive Lead amenities, access, flow, and wayfinding. -

Stem Cell Strategy by Establishing the Till & Mcculloch Medicines of Tomorrow Innovation Fund
A Pre-Budget Submission to the House of Commons Standing Committee on Finance To Implement the Canadian Stem Cell Strategy By Establishing The Till & McCulloch Medicines of Tomorrow Innovation Fund James Price President & CEO Canadian Stem Cell Foundation February 9, 2016 EXECUTIVE SUMMARY Stem cells represent the biggest innovation in medicine of the last half century. These cells have the power to cure many diseases for which current medical practice can only provide symptomatic relief and chronic care – a reality that is straining health care systems in Canada and in countries around the globe. Stem cells are a hallmark of Canadian innovation. They were first discovered in Canada and Canada is one of the top three countries in stem cell R&D, with our scientists ranking among the best in the world. Recent investments, such as the Government’s $20-million commitment to establish a cell-manufacturing facility in Toronto and the $114-million Medicine By Design grant for the University of Toronto, will help Canada move forward. However, major commitments by competitor jurisdictions – most notably California, with its investment of $3 billion, and Japan, with an investment of $1 billion in stem cells and regenerative medicine -- challenge Canada’s leadership in this sector of the knowledge economy. Moreover, Canada lacks a national plan to succeed in the coming cell therapy and regenerative medicine boom. The Canadian Stem Cell Strategy -- created in consultation with 150 scientists, medical doctors, leaders from major health charities, industry experts, investors and philanthropists – will: • deliver up to 10 new curative therapies within 10 years; • transform health care and ease the strain on the health system; and • attract private investment and generate 12,000 jobs for Canadians. -

(DMOH) Toronto General Hospital, University Health Network
General & Consultative Academic Hematologist, Division of Medical Oncology and Hematology (DMOH) Toronto General Hospital, University Health Network The Department of Medicine, Faculty of Medicine at the University of Toronto and the Division of Medical Oncology and Hematology at the University Health Network (UHN) are seeking to recruit a general academic hematologist for the Toronto General Hospital’s Blood Disorders Program. The successful candidate will have an academic position of Clinician Investigator and must be eligible for a full-time clinical academic appointment at the rank of Lecturer or Assistant Professor at the University of Toronto. Effective start date is September 1, 2017, or shortly thereafter. The position is well suited to early career physicians with an interest in non- and pre-malignant hematology. Strong collaborative skills are required as there is an opportunity to form closer links with the malignant hematology clinics and with our principal referring internal stakeholders. The role will be primarily ambulatory clinic based, serving hematological complications from UHN’s Cardiac, Multi-Organ Transplant and Internal Medicine programs, as well as the local community. The candidate should have a strong academic interest in postgraduate MD teaching or in quality improvement/patient safety and uphold standards of excellence. Additional qualifications in these domains are preferred. The Blood Disorders Program is a core program within the Division of Medical Oncology & Hematology at the University Health Network. As one of the top health networks in the world, the University Health Network encompasses both the Princess Margaret Cancer Centre as well as the Toronto General Hospital along with each of their respective world-class research institutes: The Ontario Cancer Institute and Toronto General Research Institute. -

Scientific Advisory Committee on Oncology Therapies (SAC-OT)
Scientific Advisory Committee on Oncology Therapies (SAC-OT) Membership List Core Members Alexander H.G. Paterson, MD, FRCP, FACP, MBChB (Chair) Medical Oncologist, Department of Medical Oncology, Tom Baker Cancer Centre Professor, Department of Medicine and Oncology, University of Calgary Calgary, Alberta Biography: Alexander Paterson graduated Medicine from Edinburgh University, United Kingdom in 1977 and subsequently trained at St. Bartholomew’s Hospital and Royal Marsden, London, England. He is a Fellow of the Royal College of Physicians and has been a Medical Oncologist at the Tom Baker Cancer Centre in Calgary since 1990. He is also Professor in the Departments of Medicine and Oncology at the University of Calgary, Alberta since 1995. He is a member of the Alberta Out-of-Province/Country Health Services Appeal Panel and the Board of Directors of the National Surgical Adjuvant Breast and Bowel Project (NSAPB). He is also Chair of the NSABP Protocol B-34 and the Alberta Breast Cancer Programme. He has published over 100 articles, authored over 15 book chapters, is the Editor of Fundamental Problems in Breast Cancer (I and II) and contributes a regular column to Alberta Doctors’ Digest. He has given over 200 invited lectures. Rick Abbott, BScPharm, RPEBC Pharmacy Manager, Provincial Systemic Therapy, Dr. H. Bliss Murphy Cancer Centre, Eastern Health St. John’s, Newfoundland and Labrador Biography: Rick Abbott graduated from the School of Pharmacy in 1990 at the Memorial University of Newfoundland, St. John’s, Newfoundland and Labrador. He has been the Pharmacy Manager of the Provincial Systemic Therapy since 2002 and is actively involved in the Pharmacy Profession. -

By the Numbers Excellence, Innovation, Leadership: Research at the University of Toronto a Powerful Partnership
BY THE NUMBERS EXCELLENCE, INNOVATION, LEADERSHIP: RESEARCH AT THE UNIVERSITY OF TORONTO A POWERFUL PARTNERSHIP The combination of U of T and the 10 partner hospitals affiliated with the university creates one of the world’s largest and most innovative health research forces. More than 1,900 researchers and over 4,000 graduate students and postdoctoral fellows pursue the next vital steps in every area of health research imaginable. UNIVERSITY OF TORONTO Sunnybrook Health St. Michaelʼs Sciences Centre Hospital Womenʼs College Bloorview Kids Hospital Rehab A POWERFUL PARTNERSHIP Baycrest Mount Sinai Hospital The Hospital University Health for Sick Children Network* Centre for Toronto Addiction and Rehabilitation Mental Health Institute *Composed of Toronto General, Toronto Western and Princess Margaret Hospitals 1 UNIVERSITY OF TORONTO FACULTY EXCELLENCE U of T researchers consistently win more prestigious awards than any other Canadian university. See the end of this booklet for a detailed list of awards and honours received by our faculty in the last three years. Faculty Honours (1980-2009) University of Toronto compared to awards held at other Canadian universities International American Academy of Arts & Sciences* Gairdner International Award Guggenheim Fellows National Academies** Royal Society Fellows Sloan Research Fellows American Association for the Advancement of Science* ISI Highly-Cited Researchers*** 0 20 40 60 801 00 Percentage National Steacie Prize Molson Prize Federal Granting Councilsʼ Highest Awards**** Killam Prize Steacie -

Printable List of Laureates
Laureates of the Canadian Medical Hall of Fame A E Maude Abbott MD* (1994) Connie J. Eaves PhD (2019) Albert Aguayo MD(2011) John Evans MD* (2000) Oswald Avery MD (2004) F B Ray Farquharson MD* (1998) Elizabeth Bagshaw MD* (2007) Hon. Sylvia Fedoruk MA* (2009) Sir Frederick Banting MD* (1994) William Feindel MD PhD* (2003) Henry Barnett MD* (1995) B. Brett Finlay PhD (2018) Murray Barr MD* (1998) C. Miller Fisher MD* (1998) Charles Beer PhD* (1997) James FitzGerald MD PhD* (2004) Bernard Belleau PhD* (2000) Claude Fortier MD* (1998) Philip B. Berger MD (2018) Terry Fox* (2012) Michel G. Bergeron MD (2017) Armand Frappier MD* (2012) Alan Bernstein PhD (2015) Clarke Fraser MD PhD* (2012) Charles H. Best MD PhD* (1994) Henry Friesen MD (2001) Norman Bethune MD* (1998) John Bienenstock MD (2011) G Wilfred G. Bigelow MD* (1997) William Gallie MD* (2001) Michael Bliss PhD* (2016) Jacques Genest MD* (1994) Roberta Bondar MD PhD (1998) Gustave Gingras MD* (1998) John Bradley MD* (2001) Phil Gold MD PhD (2010) Henri Breault MD* (1997) Richard G. Goldbloom MD (2017) G. Malcolm Brown PhD* (2000) Jean Gray MD (2020) John Symonds Lyon Browne MD PhD* (1994) Wilfred Grenfell MD* (1997) Alan Burton PhD* (2010) Gordon Guyatt MD (2016) C H G. Brock Chisholm MD (2019) Vladimir Hachinski MD (2018) Harvey Max Chochnov, MD PhD (2020) Antoine Hakim MD PhD (2013) Bruce Chown MD* (1995) Justice Emmett Hall* (2017) Michel Chrétien MD (2017) Judith G. Hall MD (2015) William A. Cochrane MD* (2010) Michael R. Hayden MD PhD (2017) May Cohen MD (2016) Donald O. -

Annual Report 2010-2011
ON TARIO IN STIT U T E FOR C A N C E R RE S Ontario Institute for E ARC H ANNU Cancer Research A L REP ANNUAL REPORT 2010/11 ORT 2010/11 MaRS Centre, South Tower 101 College Street Suite 800 Toronto, Ontario Canada M5G 0A3 Telephone 416.977.7599 Toll-free 1.866.678.6427 [email protected] www.oicr.on.ca Funding for the Ontario Institute for Cancer Research is provided by the Government of Ontario. CJ23389 Cover.indd 1 11-07-07 6:56 PM Message from the Minister of Research and Innovation It is my pleasure to thank the Ontario Institute for Cancer Research (OICR) for the outstanding work it has done over For information about the the past year to improve the health and wellbeing of the people of Ontario. Ontario Institute for Cancer Research please contact: T he work you do has a direct impact on the lives of everyone in the province. It offers hope to the nearly 70,000 Ontarians Rhea Cohen who will be diagnosed with cancer in 2011 and to many millions more around the world. T he survival rate for most types Director of Communications [email protected] of cancer is constantly improving – and your work is helping to make that happen. 416-673-6642 OICR is recognized around the world for its leadership in cancer research. T he Institute’s high profile role – and that of its President and Scientific Director, Dr. Tom Hudson – in the International Cancer Genome Consortium is just one example of that leadership. -

CSHL AR 1977.Pdf
ANNUAL REPORT 1977 COLD SPRING HARBOR, NEW YORK COLD SPRING HARBOR LABORATORY COLD SPRING HARBOR, LONG ISLAND, NEW YORK OFFICERS OF THE CORPORATION Dr. Harry Eagle, Chairman Edward Pulling, Vice-Chairman Dr. Bayard Clarkson, Secretary Clarence E. Galston, Treasurer William R. Udry, Assistant Secretary-Treasurer Dr. James D. Watson, Laboratory Director William R. Udry, Administrative Director BOARD OF TRUSTEES Institutional Trustees State University of New York at Stony Brook Albert Einstein College of Medicine Dr. Joseph R. Kates Dr. Harry Eagle University of Wisconsin Columbia University Dr. Julian Davies Dr. Charles R. Cantor Wawepex Society Duke University Bache Bleecker Dr. Walter Guild Harvard University Individual Trustees School of Public Health Dr. Howard Hiatt Emilio G. Collado Long Island Biological Association Robert L. Cummings Norris Darrell Edward Pulling Walter N. Frank, Jr. Massachusetts Institute of Technology Clarence E. Galston Dr. Herman Eisen William R. Grant New York University Medical Center Mrs. Robert H. P. Olney Dr. Vittorio Defendi Walter H. Page William S. Robertson Princeton University Mrs. Franz Schneider Dr. Arnold J. Levine Dr. James D. Watson The Rockefeller University Dr. Rollin Hotchkiss Honorary trustees Sloan-Kettering Institute Dr. Alexander Hollaender Dr. Bayard Clarkson Dr. H. Bentley Glass Officers and trustees listed are as of December 31,1977. DIRECTOR'S REPORT When I was a boy in Chicago, the scientist was a poorlyabout that we are closet big businessmen who need to paid, absent-minded dreamer, very bright,if not abe kept in check by massive regulations, if not the threat genius, and culturally destined never to lead the masses, of jail. -
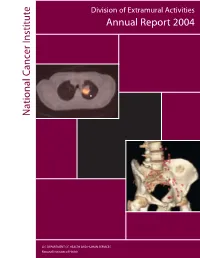
National C Ancer Institute Annual Report 2004
Division of Extramural Activities Annual Report 2004 National Cancer Institute National Cancer U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health Molecular Imaging for Cancer The NCI has made a significant committment to support the discovery, development, and delivery of cutting- edge molecular imaging agents and technologies. The field of molecular imaging has grown rapidly in response to this committment, and the applications being developed will have a far-reaching impact on the detection, diagnosis, and treatment of cancer patients. The images on the cover demonstrate the power of two different types of molecular imaging techniques applied to cancer detection. The image on the top is generated using positron emission tomography-computed tomog- raphy (PET-CT) fusion imaging (image reproduced with permission from David W. Townsend, Ph.D., Department of Radiology, University of Tennessee). Computed-tomography (CT) alone, a technique based on the use of x-rays, is capable of generating images with exquisite anatomical detail, but does not provide the clinician with physiologic information about lesions that are detected. Conversely, positron emission tomogra- phy (PET) alone, a technique based on the use of radiolabeled imaging agents, provides the clinician with func- tional information about whether a lesion is cancerous or not, but the resulting images do not contain the anatomic detail necessary to determine exact location within the body. In the image shown, the radiolabeled agent being used is 2-[18F]fluoro-2-deoxy-D-glucose ([18F] FDG), an agent that reports on the increased metabolism occuring in a cancer cell. In the past, these two types of images were acquired using two sepa- rate machines at two different times.