Dough Reduction Chemistry a Guide to Reducing Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperbranched Polyaspartate Esters and a Process for Their Preparation
Europäisches Patentamt *EP000743335B1* (19) European Patent Office Office européen des brevets (11) EP 0 743 335 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C08G 63/685 of the grant of the patent: 26.09.2001 Bulletin 2001/39 (21) Application number: 96107069.5 (22) Date of filing: 06.05.1996 (54) Hyperbranched polyaspartate esters and a process for their preparation Hyperverzweigte Polyaspartatester und Verfahren zu ihrer Herstellung Ester de polyaspartate hyperramifiés et procédé pour leur préparation (84) Designated Contracting States: (74) Representative: Pettrich, Klaus-Günter, Dr. AT BE CH DE ES FR GB IT LI NL SE c/o Bayer AG, Konzernbereich RP (30) Priority: 18.05.1995 US 443505 Patente und Lizenzen 51368 Leverkusen (DE) (43) Date of publication of application: 20.11.1996 Bulletin 1996/47 (56) References cited: US-A- 5 126 170 (73) Proprietor: Bayer Corporation Pittsburgh, PA 15205-9741 (US) • ISRA L JOURNAL OF CHEMISTRY, vol. 9, 1971, JERUSALEM, pages 105-109, XP000670606 A. (72) Inventors: SINGERMAN ET AL.: "Poly threo-beta-hydroxy • Yeske, Philip E. aspartic acid" Pittsburgh, PA 15228 (US) • Gindin, Lyuba K. Remarks: Pittsburgh, PA 15216 (US) The file contains technical information submitted • Wicks, Douglas A. after the application was filed and not included in this Mt. Lebanon, PA 15228 (US) specification • Jonsson, E. Haakan Coraopolis, PA 15108 (US) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. -

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17
Document: 620-TDS-119 TECHNICAL DATA SHEET Revision: 4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17 Product Description As a dough conditioner, SAF Pro® Croustilis® reduces the formation of blisters and enables the successful production of bakery products. Regulatory Product is produced from materials that are wholesome and free from extraneous matter. Product is prepared following Good Manufacturing Practices, and complies with the Federal Food, Drug and Cosmetic Act of 1938, as amended and published in the CFR. Lesaffre Yeast Corporation is the US importer of record for SAF Pro Dough Conditioners manufactured by LIS- France, Cerences, France. LIS-France is included in Lesaffre Yeast Corporation’s Foreign Supplier Verification Program. Ingredient Statement Enriched Wheat Flour, Monoglycerides of Fatty Acids, Ascorbic Acid, Enzymes Manufacturing Process Blending, packaging Applications Baguettes, bagels, pizza crusts, mixes, frozen dough, par baked, retarded doughs, no-time doughs Usage Add directly to the flour at 0.3 – 0.5% of the total flour weight Item Numbers / Packaging Format / Grind / UPC Item Net Wt. Package Form UPC - Case Number 27201 10 kg. Poly-lined corrugated box Powder 0 17929 27201 2 Import / Export Tariff code 2106.90.99.98 Product Specifications Parameter Specification Test Method Ascorbic Acid 2.16-2.64% Internal Method Phc022 Salmonella Negative / 25 gm SMS (BIOMERIEUX), validated AFNOR # AES 10/04 - 05/04 7475 West Main St. Milwaukee, WI 53214 Document: 620-TDS-119 TECHNICAL DATA SHEET Revision: 4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17 Package / Pallet Dimensions Item Case Pallets L W H Cu. Tiers Units/ Cases/ L W H Cu. -

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
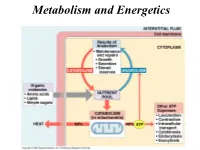
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Thermal Decomposition of the Amino Acids Glycine, Cysteine, Aspartic Acid, Asparagine, Glutamic Acid, Glutamine, Arginine and Histidine
bioRxiv preprint doi: https://doi.org/10.1101/119123; this version posted March 22, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine Ingrid M. Weiss*, Christina Muth, Robert Drumm & Helmut O.K. Kirchner INM-Leibniz Institute for New Materials, Campus D2 2, D-66123 Saarbruecken Germany *Present address: Universität Stuttgart, Institut für Biomaterialien und biomolekulare Systeme, Pfaffenwaldring 57, D-70569 Stuttgart, Germany Abstract Calorimetry, thermogravimetry and mass spectrometry were used to follow the thermal decomposition of the eight amino acids G, C, D, N, E, Q, R and H between 185°C and 280°C. Endothermic heats of decomposition between 72 and 151 kJ/mol are needed to form 12 to 70 % volatile products. This process is neither melting nor sublimation. With exception of cysteine they emit mainly H2O, some NH3 and no CO2. Cysteine produces CO2 and little else. The reactions are described by polynomials, AA → a (NH3) + b (H2O) + c (CO2) + d (H2S) + e (residue), with integer or half integer coefficients. The solid monomolecular residues are rich in peptide bonds. 1. Motivation Amino acids might have been synthesized under prebiological conditions on earth or deposited on earth from interstellar space, where they have been found [Follmann and Brownson, 2009]. Robustness of amino acids against extreme conditions is required for early occurrence, but little is known about their nonbiological thermal destruction. There is hope that one might learn something about the molecules needed in synthesis from the products found in decomposition. -

Analysis of the Effects of Three Commercially Available Supplements on Performance, Exercise Induced Changes and Bio-Markers in Recreationally Trained Young Males
Analysis of the effects of three commercially available supplements on performance, exercise induced changes and bio-markers in recreationally trained young males Robert Cooper A thesis is submitted in partial fulfilment of the requirements of the University of Greenwich for the Degree of Doctor of Philosophy This research programme was carried out in collaboration with GlaxoSmithKline Maxinutrition division December 2013 School of Science University of Greenwich, Medway Campus Chatham Maritime, Kent ME4 4TB, UK i DECLARATION “I certify that this work has not been accepted in substance for any degree, and is not concurrently being submitted for any degree other than that of Doctor of Philosophy being studied at the University of Greenwich. I also declare that this work is the result of my own investigations except where otherwise identified by references and that I have not plagiarised the work of others”. Signed Date Mr Robert Cooper (Candidate) …………………………………………………………………………………………………………………………… PhD Supervisors Signed Date Dr Fernando Naclerio (1st supervisor) Signed Date Dr Mark Goss-Sampson (2nd supervisor) ii ACKNOWLEDGEMENTS Thank you to my supervisory team, Dr Fernando Naclerio, Dr Mark Goss Sampson and Dr Judith Allgrove for their support and guidance throughout my PhD. Particular thanks to Dr Fernando Naclerio for his tireless efforts, guidance and support in developing the research and my own research and communication skills. Thank you to Dr Eneko Larumbe Zabala for the statistics support. I would like to take this opportunity to thank my wonderful mother and sister who continue to give me the support and drive to succeed. Also on a personal level thank you to my amazing fiancée, Jennie Swift. -

Calcium Peroxide (CP) Azodicarbonamide (ADA)
Enzymatic Solutions to replace chemicals in wheat flour treatments . Calcium Peroxide (CP) . Azodicarbonamide (ADA) Norman Loop Mühlenchemie GmbH & Co. KG Ahrensburg, Germany LP04012001 Agenda Muehlenchemie Chemical oxidants in flour milling industry Regulatory / Legal status How to replace them? Practical Examples . Calcium Peroxide (CP) Replacement . Azodicarbonamide (ADA) Replacement Conclusion Mühlenchemie: the Flour Company We are an innovative enterprise – present in the market for over 90 years: Mühlenchemie makes good flours even better. Established: 1923 Domicile: Ahrensburg / Hamburg Parent company: Stern-Wywiol Gruppe, Hamburg Specialization: . Flour improvers . Enzymes . Vitamin and mineral premixes . Applications consultancy . Analytical service . Training courses and seminars Mühlenchemie: the Flour Company Market position: World market leader in flour improvers Turnover: approx 150 mill. USD Sales: In more than 110 countries globally Production facilities: Germany, China, India, Mexico, Turkey,USA and Malaysia Our dedication to millers: The FlourWorld Museum: a collection of more than 3,000 flour sacks from the milling industry Chemical oxidants in flour milling industry “Would you eat your yoga mat?” Oxidizing Agents as Flour Improvers Azodicarbonamide (ADA) Calcium peroxide (CP) Chlorine & chlorine dioxide Benzoyl peroxide (BPO) Potassium Bromate 7 Calcium Peroxide (CP) Azodicarbonamide (ADA) Slow oxidizing effect Fast oxidizing effect Improves dough handling properties Improves dough stability (drying effect) Improves -

Potassium Bromate
POTASSIUM BROMATE VWR International, Pty Ltd Chemwatch: 1484 Issue Date: 25/01/2013 Version No: 6.1.1.1 Print Date: 10/12/2013 Safety Data Sheet according to WHS and ADG requirements S.GHS.AUS.EN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING Product Identifier Product name POTASSIUM BROMATE Chemical Name potassium bromate Synonyms Br-K-O3, KBrO3, bromic acid potassium salt Proper shipping name POTASSIUM BROMATE Chemical formula BrHO3.K Other means of identification Not Available CAS number 7758-01-2 Relevant identified uses of the substance or mixture and uses advised against Used as laboratory reagent, oxidising agent, permanent wave compound, maturing agent in flour, dough conditioner and food additive. Bromate is Relevant identified uses converted to bromide in the baking or cooking process, but the levels are not in excess of the natural bromide content of many natural foods., Note: Food additive uses restricted as to proportions used., [~Intermediate ~] Details of the supplier of the safety data sheet Registered company name VWR International, Pty Ltd Unit 1/31 Archimedes Place 4172 QLD Address Australia Telephone 61 7 3009 4100 ; 1300 727 696 Fax 61 7 3009 4199 ; 1300 135 123 Website http://au.vwr.com Email [email protected] Emergency telephone number Association / Organisation Not Available Emergency telephone numbers 61 7 3009 4100 ; 1300 727 696 Other emergency telephone numbers 61 7 3009 4100 ; 1300 727 696 SECTION 2 HAZARDS IDENTIFICATION Classification of the substance or mixture HAZARDOUS CHEMICAL. DANGEROUS GOODS. According to the Model WHS Regulations and the ADG Code. -

1 Isolation and Characterization of Cyclotides from Brazilian Psychotria
Isolation and Characterization of Cyclotides from Brazilian Psychotria: Significance in Plant Defense and Co-occurrence with Antioxidant Alkaloids Hélio N. Matsuura,† Aaron G. Poth,‡ Anna C. A. Yendo,† Arthur G. Fett-Neto,† and David J. Craik‡,* †Center for Biotechnology and Department of Botany, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil ‡Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD, Australia. 1 ABSTRACT Plants from the genus Psychotria include species bearing cyclotides and/or alkaloids. The elucidation of factors affecting the metabolism of these molecules as well as their activities may help to understand their ecological function. In the present study, high concentrations of antioxidant indole alkaloids were found to co-occur with cyclotides in Psychotria leiocarpa and P. brachyceras. The concentrations of the major cyclotides and alkaloids in P. leiocarpa and P. brachyceras were monitored following herbivore- and pathogen-associated challenges, revealing a constitutive, phytoanticipin-like accumulation pattern. Psyleio A, the most abundant cyclotide found in the leaves of P. leiocarpa, and also found in P. brachyceras leaves, exhibited insecticidal activity against Helicoverpa armigera larvae. Addition of ethanol in the vehicle for peptide solubilization in larval feeding trials proved deleterious to insecticidal activity, and resulted in increased rates of larval survival in treatments containing indole alkaloids. This suggests that plant alkaloids ingested by larvae might contribute to herbivore oxidative stress detoxification, corroborating, in a heterologous system with artificial oxidative stress stimulation, the antioxidant efficiency of Psychotria alkaloids previously observed in planta. Overall, the present study reports data for eight novel cyclotides, the identification of P. -

Abstract Measurement and Analysis of Bromate Ion
ABSTRACT MEASUREMENT AND ANALYSIS OF BROMATE ION REDUCTION IN SYNTHETIC GASTRIC JUICE by Jason Dimitrius Keith Bromate ion is a possible carcinogen that is regulated by the US EPA at a Maximum Contamination Level (MCL) of 10 µg/L in drinking water. In order to propose an improved scientifically appropriate bromate ion MCL, a more rigorous scientific methodology is needed for determining low level dose health risks. The objectives of this research project were to measure bromate ion with oxidizing and/or reducing agents typically ingested in foods and drinking water. The loss of bromate ion in HCl is too slow for significant reduction in the stomach. -5 Addition of 10 M H2S, a gastric juice component, decreases the half-life from 153 to 14 minutes. The ingested reducing agents iodide ion, nitrite ion, and iron(II) decrease the lifetime of bromate ion in the stomach. Chlorine, monochloramine, and iron(III) have little actual effect on the lifetime of bromate ion. The measured rates and chemical details of the reactions are discussed. MEASUREMENT AND ANALYSIS OF BROMATE ION REDUCTION IN SYNTHETIC GASTRIC JUICE A Thesis Submitted to the faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of Chemistry by Jason Dimitrius Keith Miami University Oxford, Ohio 2005 Co-Advisor________________ (Dr. Gilbert Gordon) Co-Advisor________________ (Dr. Gilbert E. Pacey) Reader_________________ (Dr. Michael W. Crowder) Reader_________________ (Dr. Hongcai Zhou) TABLE OF CONTENTS TABLE OF CONTENTS ii LIST OF TABLES iii LIST OF FIGURES iv ACKNOWLEDGEMENTS v INTRODUCTION 1 Bromate Ion Chemistry and Human Toxicology 1 Prior Analytical Methodology 6 Objectives 7 METHOD DEVELOPMENT AND ESTABLISHMENT OF PROTOCOLS 7 Solution Preparation 7 Preparation and Measurement of Stock HOCl/ Cl2 and ClNH2 Solutions 11 Measurement of Iron(II) and Iron(III) in Solution 12 Instrumentation. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride.