Re-Introduction of Vivax Malaria in a Temperate Area (Moscow Region, Russia): a Geographic Investigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moscow United Electric Grid Company” As of July 13, 2007 (Minutes No.46 As of July 17, 2007)
Approved by the Board of Directors decision of Open Joint-Stock Company “Moscow United Electric Grid Company” as of July 13, 2007 (Minutes No.46 as of July 17, 2007) Amendments No. 1 to the Charter Open Joint-Stock Company “Moscow United Electric Grid Company” To introduce the following amendments in the Charter of Open Joint-Stock Company “Moscow United Electric Grid Company”: To state the Company List of Branches (Appendix No. 1 to the Charter) as follows: List of Branches OJSC “Moscow United Electric Grid Company” No. Name Address 1. Central Electric Networks 115201, Moscow city, Kashirskoye highway, 18 2. Southern Electric Networks 115201, Moscow city, Kashirskoye highway, 18 3. Eastern Electric Networks 107140, Moscow city, Nizhnyaya Krasnoselskaya street, 6, bld. 1 4. Oktyabrskie Electric Netwowrks 127254, Moscow city, Rustaveli street, 2 5. Northern Electric Networks 141070, Moscow region, Korolev city, Gagarina street, 4 6. Noginsk Electric Networks 142400, Moscow region, Noginsk city, Radchenko street, 13 7. Podolsk Electric Networks 142117, Moscow region, Podolsk city, Kirova street, 65 8. Kolomna Electric Networks 140408, Moscow region, Kolomna city, Oktyabrskoy Revolyutsii street, 381а 9. Shatura Electric Networks 140700, Moscow region, Shatura city, Sportivnaya street, 12 10. Western Electric Networks 121170, Moscow city, 1812 Goda street, estate 15 11. Kashira Electric Networks 142900, Moscow region, Kashira city, Klubnaya street, 4 12. Mozhaisk Electric Networks 143200, Moscow region, Mozhaisk city, Mira street, 107 13. Dmitrov Electric Networks 141800, Moscow region, Dmitrov city, Kosmonavtov street, 46 14. Volokolamsk Electric Networks 143600, Moscow region, Volokolamsk city, Novosoldatskaya street, 58 15. Moskabelenergoremont (MKER) 115569, Moscow city, Shipilovskaya street, 13, bld. -
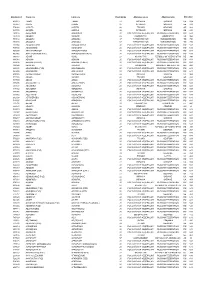
FÁK Állomáskódok
Állomáskód Orosz név Latin név Vasút kódja Államnév orosz Államnév latin Államkód 406513 1 МАЯ 1 MAIA 22 УКРАИНА UKRAINE UA 804 085827 ААКРЕ AAKRE 26 ЭСТОНИЯ ESTONIA EE 233 574066 ААПСТА AAPSTA 28 ГРУЗИЯ GEORGIA GE 268 085780 ААРДЛА AARDLA 26 ЭСТОНИЯ ESTONIA EE 233 269116 АБАБКОВО ABABKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 737139 АБАДАН ABADAN 29 УЗБЕКИСТАН UZBEKISTAN UZ 860 753112 АБАДАН-I ABADAN-I 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 753108 АБАДАН-II ABADAN-II 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 535004 АБАДЗЕХСКАЯ ABADZEHSKAIA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 795736 АБАЕВСКИЙ ABAEVSKII 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 864300 АБАГУР-ЛЕСНОЙ ABAGUR-LESNOI 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 865065 АБАГУРОВСКИЙ (РЗД) ABAGUROVSKII (RZD) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 699767 АБАИЛ ABAIL 27 КАЗАХСТАН REPUBLIC OF KAZAKHSTAN KZ 398 888004 АБАКАН ABAKAN 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 888108 АБАКАН (ПЕРЕВ.) ABAKAN (PEREV.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 398904 АБАКЛИЯ ABAKLIIA 23 МОЛДАВИЯ MOLDOVA, REPUBLIC OF MD 498 889401 АБАКУМОВКА (РЗД) ABAKUMOVKA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 882309 АБАЛАКОВО ABALAKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 408006 АБАМЕЛИКОВО ABAMELIKOVO 22 УКРАИНА UKRAINE UA 804 571706 АБАША ABASHA 28 ГРУЗИЯ GEORGIA GE 268 887500 АБАЗА ABAZA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 887406 АБАЗА (ЭКСП.) ABAZA (EKSP.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 -

Russian Museums Visit More Than 80 Million Visitors, 1/3 of Who Are Visitors Under 18
Moscow 4 There are more than 3000 museums (and about 72 000 museum workers) in Russian Moscow region 92 Federation, not including school and company museums. Every year Russian museums visit more than 80 million visitors, 1/3 of who are visitors under 18 There are about 650 individual and institutional members in ICOM Russia. During two last St. Petersburg 117 years ICOM Russia membership was rapidly increasing more than 20% (or about 100 new members) a year Northwestern region 160 You will find the information aboutICOM Russia members in this book. All members (individual and institutional) are divided in two big groups – Museums which are institutional members of ICOM or are represented by individual members and Organizations. All the museums in this book are distributed by regional principle. Organizations are structured in profile groups Central region 192 Volga river region 224 Many thanks to all the museums who offered their help and assistance in the making of this collection South of Russia 258 Special thanks to Urals 270 Museum creation and consulting Culture heritage security in Russia with 3M(tm)Novec(tm)1230 Siberia and Far East 284 © ICOM Russia, 2012 Organizations 322 © K. Novokhatko, A. Gnedovsky, N. Kazantseva, O. Guzewska – compiling, translation, editing, 2012 [email protected] www.icom.org.ru © Leo Tolstoy museum-estate “Yasnaya Polyana”, design, 2012 Moscow MOSCOW A. N. SCRiAbiN MEMORiAl Capital of Russia. Major political, economic, cultural, scientific, religious, financial, educational, and transportation center of Russia and the continent MUSEUM Highlights: First reference to Moscow dates from 1147 when Moscow was already a pretty big town. -

MEGA Khimki Tver Region Market Overview Welcome
MEGA Khimki Tver region Market overview Welcome Dmitrov L e y n Sergiev-Posad Catchment areas People Distance i w y n h to MEGA Khimki Klin g w r a e h V Vladimir d o ol s e o k ko k o region la o s k m e v s Pushkin s Mytischi ko h o av e w r sl t Schelkovo y i o h . r a w m y Y Primary 398,200 < 17 km D Zheleznodorozhny M K A Smolensk Moscow D Balashikha region Podolsk Naro-Fominsk Secondary 1,424,200 17–40 km Krasnogorsk y Klimovsk v hw hw uziasto oe y nt RUSSIA’S FIRST IKEA WAS OPENED IN sk E Obninsk izh Kolomna or Reutov Tertiary 3,150,656 40–140 km ov KHIMKI IN 2000. MEGA KHIMKI SOON N Serpukhov FOLLOWED IN 2004 AND BECAME THE Kaluga region LARGEST RETAIL COMPLEX IN RUSSIA Tula region Total area: 4,973,000 AT THE TIME. Odintsovo N o v o ry y a hw z e a ko n s sk Min o e wy h h w oe y vsk Kie Despite several new retail centres opening their doors along the Leningradskoe Shosse, y y w w h MEGA Khimki remains one of the district’s h e oe o sk k most popular shopping destinations, largely s h Troitsk z Scherbinka v u a al due to its location, well-designed layout and K h s r retail mix. a V Domodedovo New tenants and constant improvements to the centre have significantly increased customer numbers. -

Vikline® Complex Service in Foreign Trade
VIKLINE® COMPLEX SERVICE IN FOREIGN TRADE ® VIKLINE 2021 ABOUT VIKLINE® VIKLINE® bringing together today's leaders in customs clearance & logistics, since 2007 VIKLINE® is: ViK Line Company LLC customs representative offering comprehensive services for clearance of goods for all regions of the Russian Federation (license № 0932/01 from 15.11.2018) JSC «Logistics centre «WEST GATE» temporary storage warehouse (certifacate № 10013/260214/10130/6 from 04.06.2021). customs warehouse (certificate №10013/041/А from 22.05.2020) «Logistics centre «WEST GATE» is located in Mozhaysk District of Moscow Region (Otyakovo village, 103,5 km of M1 highway) KTM LLC importer of fresh fruits and vegetables www.vikline.ru | +7 (495) 983 59 74 | [email protected] OUR SERVICES VIKLINE® Temporary storage warehouse Customs representative Customs warehouse services Air cargos, excise cargos, Transport & sea cargos logistics Branch solutions: live animals & plants, Certification food, consumer goods, industrial equipment etc Personal online office www.vikline.ru | +7 (495) 983 59 74 | [email protected] OUR GEOGRAPHY VIKLINE® Saint Petersburg Moscow We offer customs clearance in the most popular Mozhaysk Bryansk logistics areas! Zabaikalsk Novorossiysk Vladivostok Nahodka Main locations Additional locations VIKLINE® ALL-RUSSIAN CUSTOMS REPRESENTATIVE Complete range of customs and logistics services License № 0932/01 from 15.11.2018 Head quarter in Moscow: 8/1 Molodogvardeiskaya st. (9:00 - 18:00 Mon to Fri) Help deck: 08:00 - 20:00 daily 93% DAY-TO-DAY CUSTOMS CLEARANCE! customs clearance in 2 days - 1% customs clearance in 24 hours - 6% www.vikline.ru | +7 (495) 983 59 74 | [email protected] OUR PROFILE VIKLINE® EQUIPMENT VIKLINE® Customs representative has many years of practice and experience in customs clearance of goods of daily demand. -

Methods of Statistical Estimation of Circular Migration and Formal And
Statistical Journal of the IAOS 36 (2020) 535–547 535 DOI 10.3233/SJI-190604 IOS Press Methods of statistical estimation of circular migration and formal and informal employment in the Moscow agglomeration based on the integration of various data sources Polina Kriuchkovaa;b, Filipp Sleznovc;d;∗, Denis Fomchenkoc, Vladimir Laikamc and Igor Zakharchenkovc aThe Department of Economic Policy and Development, Moscow City Government, Moscow, Russia bHigher School of Economics, The National Research University, Moscow, Russia cThe Analytical Center of Moscow Government, Moscow, Russia dLomonosov Moscow State University, Moscow, Russia Abstract. Assessing circular migration, formal and informal employment and its spatiotemporal characteristics is a complex methodological and practical task for official statistics. A combination of various data sources, including official statistics, administrative data, and data from mobile operators, may provide new opportunities for obtaining circular migration, formal and informal employment estimates for the purposes of various levels of government, including the level of city management. The purpose of this paper is to demonstrate how the use of administrative data together with the mobile operators’ data can promptly improve the accuracy and informativeness of statistical indicators of the labor market including formal and informal employment, circular migration, etc. The population and employment in Moscow and in the Moscow agglomeration are the subjects of this paper. Authors combine several data sources such as the federal administrative data from the Pension Fund of the Russian Federation and the Federal Tax Authority, data from the Moscow city online public services, data from the mobile phone operators, as well as official statistical information provided by Russian Statistic Authority. -

25 YEARS of GROWTH in HARMONY with OUR CUSTOMERS Highlights 1
ANNUAL REPORT 2016 25 YEARS OF GROWTH IN HARMONY WITH OUR CUSTOMERS Highlights 1. Strategic Report 5. For Shareholders and Investors Key Financial Performance Indicators 2. Overview of Operations 6. Sustainable Development Mission and Values 3. Financial Results Contacts 2 / 197 4. Corporate Governance System Appendices www.mkb.ru Annual Report 2016 / Table of Contents Table of Contents Highlights Key Financial Performance Indicators Mission and Values 1. Strategic Report 2. Overview of operations 5. For shareholders and investors Appendices 1.1. Address of the Chairman of the 2.1. Corporate banking 6. Sustainable development Appendix 1. Supervisory Board IFRS Statements 2.2. Retail banking 6.1. Human Resources 1.2. Address of the Chairman of the Appendix 2. Management Board 2.3. Cash handling 6.2. Corporate Culture RAS Statements and Social Responsibility 1.3. Management Responsibility 3. Financial results Appendix 3. Statement 6.3. Information technologies List of interested party transactions 3.1. Income statement analysis made in the reporting year (2016) 1.4. Economy and banking sector 6.4. Society 3.2. Key Results List of major transactions made in 1.5. Business model. 6.5. Environmental Management the reporting year (2016) Competitive advantages. 3.3. Income statement analysis Position in the industry. Contacts List of transactions requiring 3.4. Structure of assets and liabilities approval under the Charter made in 1.6. Strategy under IFRS the reporting year (2016) 1.7. Risk Management 4. Corporate governance system Appendix 4. Report on Compliance with the Principles and Recommendations of the Corporate Governance Code. Highlights 1. Strategic Report 5. -
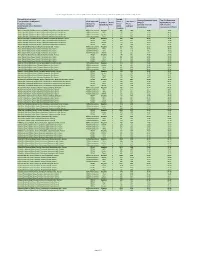
Appendix 2: Q4 (Oct-Dec) 2020 Cities in Russia Top Mobile Internet Providers Based on Average Download and Top 10% Speeds of Speedtest Intelligence Data
Appendix 2: Q4 (Oct-Dec) 2020 Cities in Russia Top Mobile Internet Providers based on average download and top 10% speeds of Speedtest Intelligence data City and Location Name Sample Average Download Speed Top 10% Download from Speedtest Intelligence / Claim Approved CoUnt / Test CoUnt / Provider / Rank / (Mbps) / Speed (Mbps) / Топ Название города, /Заявление Число Число Провайдер Ранг Средняя скорость 10% скорость местоположения из Speedtest одобрено точек замеров скачивания скачивания (Мбит/с) Intelligence замеров Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия All/Все технологии MegaFon 1 468 1369 34.89 79.85 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия All/Все технологии MTS 2 227 612 23.35 48.91 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия All/Все технологии Beeline 3 125 516 18.92 34.17 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия All/Все технологии Tele2 4 204 571 18.60 41.73 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия LTE/4G MegaFon 1 439 1257 35.61 79.35 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия LTE/4G MTS 2 205 491 24.35 49.98 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия LTE/4G Beeline 3 113 430 20.07 34.29 Abakan, Republic of Khakassia, Russia / Абакан, Республика Хакасия, Россия LTE/4G Tele2 4 192 527 19.17 43.24 AksaY, Rostov Oblast, Russia / Аксай, Ростовская обл., Россия All/Все технологии MegaFon 1 207 417 28.17 -

The Holy Trinity in Russian Spirituality the Deep Stream of Russian Spirituality Continues to Inspire the Faithful in the USSR
they have pushed me away. Only prayer is left, but my mouth is dumb with grief. All mothers of the World, and Christians, I request your prayers, your defence and help; young Timofei, an orphan yet with a living mother, stretches out his child's hands to you. The Holy Trinity in Russian Spirituality The deep stream of Russian spirituality continues to inspire the faithful in the USSR. A channel into this stream was provided by a recent article (and particu larly by its footnotes) in the official Church publication, The Journal 0'£ the Moscow Patriarchate No. I, 1975 (pp. 63-80). Written by Archbishop Pitirim of V olokalamsk and entitled "The Church as the Realization of the Trinitarian Oikonomy", this article was originally presented as a report to the Uppsala conference, "Church Days - 74", held from 30 August-3 September, 1974. The extract printed below consists chiefly of footnotes, but these offer the ,.eader many riches. A live perception of the Triune God was natural to the Russian religious con sciousness from the very beginning. It is reflected not only in the rich liturgical inheritance of the Russian Orthodox Church, common to all Eastern Orthodoxy, but in the characteristic national features of the Russian ecclesiastical conscious ness. In ancient Lives of Saints, which was the favourite reading matter and prac tically the only means of spiritually educating the people, an important place is occupied by theological talks on the triune nature of God. Despite their ab stractness they penetrated deeply the consciousness of the Russian Christian and moulded him. -

University Centre Trained 437 Students from Basic Departments of MSU, MIPT, MIREA, ®Dubna¯ University and JINR Member-States Uni- Versities
International Student Practices. Annual student The third stage (26.09Ä12.10): 33 students from training courses 2012 were held in three stages. The South Africa and 8 from Belarus conducted projects in program of the ˇrst week for all stages of training FLNR (6), FLNP (4), LIT (1), LRB (1), BLTP (1), traditionally included introductory lectures on the re- VBLHE (1), DLNP (1). search conducted in JINR laboratories. Further on, The training courses were completed by partici- students worked on the researchÄeducational projects, pants' giving report-presentations. These reports are which they had chosen. The total number of partici- available on UC web-site in training courses sections, pants in the training courses 2012 came up to 119 stu- subsection ®Events¯. dents (see Fig. 1). Educational Process on the Basis of JINR. In 2012, the University Centre trained 437 students from basic departments of MSU, MIPT, MIREA, ®Dubna¯ University and JINR Member-States uni- versities. For 31 students of MIPT, MEI, Vladimir, Gomel, St. Petersburg, Tomsk and Tula State Uni- versities, Kazan State Technological University, Kiev National University, Siberian Federal University, UC organized summer practical and undergraduate training courses. The students were trained in VBLHE (17 stu- dents), FLNP (11), DLNP (2), FLNR (1). The UC web- site (http://uc.jinr.ru/) contents of training courses data- base was upgraded (both English and Russian versions) in sections: particle physics and quantum ˇeld theory Fig. 1. The number of international training courses partici- (30 courses), nuclear physics (23), condensed matter, pants over the years physics of nanostructures and neutron physics (17), physics research facilities (15), information technolo- The participants have the opportunity to ˇnd more gies (13), mathematical and statistical physics (12). -

Invest in Moscow Region
INVEST IN MOSCOW REGION LOCATION GENERAL INFORMATION Dubna Sergiev Posad Mytishchy Population - 7.1 million Korolev Khimki Balashiha Urban population - 80% Odintsovo Lyubertsy More than 100 000 people live Zhukovsky in 20 cities of Moscow Region Podolsk Shatura Zaraysk DEVELOPED TRANSPORT INFRASTRUCTURE Road density km/1000 km2 3 international airports 232 Total passengers - 60 million people/year The total volume of cargo transportation in Russia (%) Moscow Central Federal Region District of Russia Density of railways 40 km/1000 km2 60 26 - Volume of cargo transportation in Moscow and Central Federal Moscow Region Moscow District of Russia Region QUALIFIED WORK FORCE Key Facts: 4.5 million people are 18-60 years old Salaries are 30% lower than in Moscow 71% of population has a higher education or vocational training CITIES OF MOSCOW REGION HAVE HISTORICALLY HIGH PERSONNEL POTENTIAL INNOVATIVE, HIGH-TECH HI-TECH BIOTECHNOLOGY DEVELOPMENT and SPACE ENGINEERING PHARMACEUTICALS Korolev, Podolsk, Dubna Podolsk, Kolomna, Klimovsk Pushchino, Chernogolovka, Obolensky Population Population Population 464 793 people 404 583 people 47 615 people THE LARGEST CONSUMER MARKET IN RUSSIA Tver region 30 million people live in the Moscow agglomeration or 20% of Russia's Smolensk region 300 km population Yaroslavl 1/3 of consumer spending in Russia Kaluga region region Tula region Ivanovo region Vladimir region Ryazan region ECONOMIC AND INVESTMENT INDICATORS Gross regional product of Regions of the Russian Federation (2012, billion USD) 352.57 -

Monthly Update
Russian Folk Art as Souvenirs Every person traveling around the world often questions: “what would be a good souvenirs to bring back home?” Now, of course your child is a best thing that you get from the country, but there are always things that you would like to give to your relatives and friends once you are back home. Here are several suggestions and historical background on some of most famous Russian Folk gifts that you can choose as souvenirs. Souvenirs that would be unique, beautiful and useful. You will be amazed with the variety of choice… Hohloma Hohloma wood painting is a unique ancient Russian folk craft. The birth-place of Hohloma is the forest area of Nizhny Novgorod region to the north-east of the river Volga. Hohloma is the name of a big village on the left bank of the Volga. From the earliest times masters who lived in that region made amazingly beautiful woodenware, and in the 17th century hohloma painting art was formed as a folk art phenomenon. The craft has gained world fame due to its original technique and the beauty of traditional Russian decorative patterns. Turned or carved with special chisels, articles made of limewood, are primed with clay, coated with "olifa" (boiled linseed oil), and then with powdered aluminium. After being silvered in this way, the objects are painted with refractory oil colours and then lacquered several times and tempered in ovens. The heat of 1000 makes the varnish yellow, turning "the silver" into "gold" and softening the bright colours of the painted ornament with an even golden tone.