Snail Kite Nesting Activity in the Blue Cypress Marshes During the 2000 and 2001 Breeding Seasons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
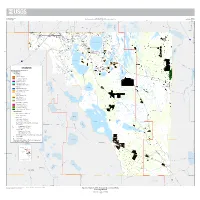
Appendix 1 U.S
U.S. Department of the Interior Prepared in cooperation with the Appendix 1 U.S. Geological Survey Florida Department of Agriculture and Consumer Services, Office of Agricultural Water Policy Open-File Report 2014−1257 81°45' 81°30' 81°15' 81°00' 80°45' 524 Jim Creek 1 Lake Hart 501 520 LAKE 17 ORANGE 417 Lake Mary Jane Saint Johns River 192 Boggy Creek 535 Shingle Creek 519 429 Lake Preston 95 17 East Lake Tohopekaliga Saint Johns River 17 Reedy Creek 28°15' Lake Lizzie Lake Winder Saint Cloud Canal ! Lake Tohopekaliga Alligator Lake 4 Saint Johns River EXPLANATION Big Bend Swamp Brick Lake Generalized land use classifications 17 for study purposes: Crabgrass Creek Land irrigated Lake Russell Lake Mattie Lake Gentry Row crops Lake Washington Peppers−184 acresLake Lowery Lake Marion Creek 192 Potatoes−3,322 acres 27 Lake Van Cantaloupes−633 acres BREVARD Lake Alfred Eggplant−151 acres All others−57 acres Lake Henry ! UnverifiedLake Haines crops−33 acres Lake Marion Saint Johns River Jane Green Creek LakeFruit Rochelle crops Cypress Lake Blueberries−41 acres Citrus groves−10,861 acres OSCEOLA Peaches−67 acresLake Fannie Lake Hamilton Field Crops Saint Johns River Field corn−292 acres Hay−234 acres Lake Hatchineha Rye grass−477 acres Lake Howard Lake 17 Seeds−619 acres 28°00' Ornamentals and grasses Ornamentals−240 acres Tree nurseries−27 acres Lake Annie Sod farms−5,643Lake Eloise acres 17 Pasture (improved)−4,575 acres Catfish Creek Land not irrigated Abandoned groves−4,916 acres Pasture−259,823 acres Lake Rosalie Water source Groundwater−18,351 acres POLK Surface water−9,106 acres Lake Kissimmee Lake Jackson Water Management Districts irrigated land totals Weohyakapka Creek Tiger Lake South Florida Groundwater−18,351 acres 441 Surface water−7,596 acres Lake Marian St. -

Kissimmee Basin Water Supply Plan (2000 KB Plan), the Planning Process Analyses Identified Key Regional Issues
TTaabbllee ooff CCoonntteennttss Appendix A: Water Supply Development Projects Water Supply Development Projects .........................................................2 Appendix B: Information for Local Government Comprehensive Plans 1. Checklist of Needed Comprehensive Plan Data........................................1 1a. Cited Statutory Provisions (relevant portions) .........................................5 2. Tables Showing which Utilities Serve which Jurisdictions ...........................8 3. Maps of Utility Areas Currently Served (2005) and to-be-Served (2025)......... 10 Appendix C: Accomplishments Overview..........................................................................................1 Appendix D: Urban and Agricultural Demand Projections Demand Assessments and Projections........................................................1 Categories of Use................................................................................2 Public Water Supply and Domestic Self-Supply Demands .................................2 Agricultural Self-Supply ...................................................................... 10 Commercial/Industrial Self-Supply ......................................................... 26 Thermoelectric Power Generation Self-Supply ........................................... 27 Recreational Self-Supply ..................................................................... 27 Total Annual Water Demand................................................................. 35 Summary of 1-in-10 Year Water -

Your Guide to Eating Fish Caught in Florida
Fish Consumption Advisories are published periodically by the Your Guide State of Florida to alert consumers about the possibility of chemically contaminated fish in Florida waters. To Eating The advisories are meant to inform the public of potential health risks of specific fish species from specific Fish Caught water bodies. In Florida February 2019 Florida Department of Health Prepared in cooperation with the Florida Department of Environmental Protection and Agriculture and Consumer Services, and the Florida Fish and Wildlife Conservation Commission 2019 Florida Fish Advisories • Table 1: Eating Guidelines for Fresh Water Fish from Florida Waters (based on mercury levels) page 1-50 • Table 2: Eating Guidelines for Marine and Estuarine Fish from Florida Waters (based on mercury levels) page 51-52 • Table 3: Eating Guidelines for species from Florida Waters with Heavy Metals (other than mercury), Dioxin, Pesticides, Polychlorinated biphenyls (PCBs), or Saxitoxin Contamination page 53-54 Eating Fish is an important part of a healthy diet. Rich in vitamins and low in fat, fish contains protein we need for strong bodies. It is also an excellent source of nutrition for proper growth and development. In fact, the American Heart Association recommends that you eat two meals of fish or seafood every week. At the same time, most Florida seafood has low to medium levels of mercury. Depending on the age of the fish, the type of fish, and the condition of the water the fish lives in, the levels of mercury found in fish are different. While mercury in rivers, creeks, ponds, and lakes can build up in some fish to levels that can be harmful, most fish caught in Florida can be eaten without harm. -

Annual Report of Activities Conducted Under the Cooperative Aquatic Plant Control Program in Florida Public Waters for Fiscal Year 2012-2013
Annual Report of Activities Conducted under the Cooperative Aquatic Plant Control Program in Florida Public Waters for Fiscal Year 2012-2013 Florida Fish and Wildlife Conservation Commission Invasive Plant Management Section Submitted by: FL Fish and Wildlife Conservation Commission Invasive Plant Management Section 3900 Commonwealth Blvd. MS705 Tallahassee, FL 32399 Phone: 850-617-9420 Fax: 850-922-1249 Annual Report of Activities Conducted under the Cooperative Aquatic Plant Control Program in Florida Public Waters for Fiscal Year 2012-2013 This report was prepared in accordance with §369.22 (7), Florida Statutes, to provide an annual summary of plants treated and funding necessary to manage aquatic plants in public waters. The Cooperative Aquatic Plant Control Program administered by the Florida Fish and Wildlife Conservation Commission (FWC) in Florida’s public waters involves complex operational and financial interactions between state, federal and local governments as well as private sector companies. FWC’s aquatic plant management program mission is to reduce negative impacts from invasive nonindigenous plants like water hyacinth, water lettuce and hydrilla to conserve the multiple uses and functions of public lakes and rivers. Invasive plants infest 96% of Florida’s 451 public waters inventoried in 2013 that comprise 1.26 million acres of fresh water. Once established, eradicating invasive plants is difficult or impossible and very expensive; therefore, continuous maintenance is critical to keep invasive plants at low levels to -

Osceola County Lakes Management Plan
2015 Osceola County Lakes Management Plan ADOPTED Osceola County BoCC September 14, 2015 Osceola County Lakes Management Plan Prepared by: Community Development Department Community Resources Office September 2015 The Mission of Osceola County’s Lakes Management Program is to protect, enhance, conserve, restore, and manage the County’s aquatic resources. We accomplish this through education, coordination with other agencies, and maintenance and management of lake systems. Activities include hydrologic management, habitat preservation and enhancement, aquatic plant management, water quality improvement, and provision of recreational opportunities. Our goal is to improve, enhance and sustain lake ecosystem health, while avoiding impacts to downstream systems, for the benefit of the fish and wildlife resources and the residents of, and visitors to, Osceola County. Cover photo: Lake Gentry CONTENTS Executive Summary ................................................................................................... 1 Section 1 Introduction ............................................................................................... 7 Section 2 Lake Management Activities .................................................................... 11 Section 3 Water Quality .......................................................................................... 20 Section 4 Lake Management Plans .......................................................................... 30 Lake Tohopekaliga .................................................................................... -
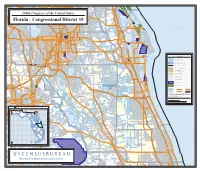
U N S U U S E U R a C S
Canaveral Natl VOLUSIA Seash Lake Dora Mount Dora O r a Midway Gopher n Wekiva River Slough g Lake Harris e Sanford B Heathrow l o Crystal o s d s Lake n o a m l John F Kennedy Lake Mary r Lake Dr Space Ctr O Tangerine T Harney 108th CongressLake Ola rl of the United States Little Lake Geneva Harris Mosquito Lagoon Lake Jessup StHwy 46 e Zellwood t Howey-in-the-Hills 6 Indian River R Astatula t 2 S 4 S tH DISTRICT 7 w Jordan Slough y 3 Turkey Lake Apopka Wekiwa Springs Longwood r ) John F Kennedy StHwy 414 a D ev Space Ctr Lake Brantley en Buck Lake (G SEMINOLE Mims 4 26 Rte St Winter Springs Puzzle Lake Max Hoeck 1 Creek StRd 46 (Main St) Forest City Casselberry Oviedo Bear Salt Lake StHwy 434 South Altamonte Fern Lake StHwyStHwy 406 StHwy 402 (Max Apopka Springs Park St Johns River StHwy 417 Chuluota 402 Brewer Memorial Pkwy) Canaveral Atlantic Ocean StHwy19 Lake Howell Mills Max Brewer Memorial Pkwy Lake Loughman Lake Natl Seash Lake Arthur Ferndale Maitland Schoolhouse Lake Lockhart Cape Rd Bear Gully Lake South StHwy 406 Lake Apopka Eaton- Clark Lake Lake Paradise Heights ville ( Garden St) W Shepherd a Fairview s Lake Lake h Banana Goldenrod i (Rouse Rd) (Rouse n Grassy DISTRICT Lake Shores 434 StHwy t) Creek Montverde Pickett S Lake Orlando Lake Osceola g th t u o 3 o n Mascotte Fox Lake S l ( A Pintail a ) v r 5 Creek t y e Lake n a 0 Lake 4 Lucy Cherry Lake Minneola Pine Winter Park e w Fairview C n y Lake ( e Sue 8 re w Hills Mills Ave 0 H StHwy 537 4 G t Lake ( 4 M DISTRICT S a y Titusville d Minneola a Lake w i r i n Starke -

Approve Agenda
Florida Greenways and Trails Council (FGTC) Public Hearing and Council Meeting PUBLIC MEETING: Thursday, January 11, 2018 TIME: 8:00 am LOCATION: Florida Sheriffs Association, Blackburn-Hunt Building, 2617 Mahan Dr, Tallahassee, FL 32308 FGTC Members in Attendance for Thursday Council Meeting: Brian Smith, Appointed by the Governor-Trail User; Sam Carr, Appointed by the Governor- Greenway User; Sarrah Glassner, (Chair) Appointed by the Governor-Greenway User; Kasey C. (KC) Ingram-Traylor, Appointed by the President of the Senate-Trail User; Chuck Barrowclough, Appointed by the President of the Senate-Greenway User; Becky Afonso, (Vice Chair) Appointed by the President of the Senate-Greenway User; Ralph Lair, Appointed by the Speaker of the House-Greenway User; Faron Boggs, Appointed by the Speaker of the House-Trail User; Sine Murray, Florida Department of Environmental Protection (FDEP); Jerrie Lindsey, Florida Fish and Wildlife Conservation Commission(FWC); Robin Birdsong, Florida Department of Transportation(FDOT); Marti Miller, Florida Department of Agriculture and Consumer Services(DACS); Patricia M. Steed, Regional Planning Council (Jennifer Codo-Salisbury attended afternoon session on behalf of Patricia M. Steed). OGT Staff in Attendance for Thursday Council Meeting: Samantha Browne, Bureau Chief; Eric Draper, Director, Florida Park Service; David Clark, Deputy Secretary of Land and Recreation; Doug Alderson, Assistant Bureau Chief, Britney Moore, Regional Land Trails Coordinator-North; Ashley Killough, Regional Land Trails Coordinator- -

Kissimmee Chain of Lakes Long-Term Management Plan Project
22001111 IInntteerraaggeennccyy DDrraafftt KKiissssiimmmmeeee CChhaaiinn ooff LLaakkeess LLoonngg--TTeerrmm MMaannaaggeemmeenntt PPllaann South Florida Water Management District Florida Fish and Wildlife Conservation Commission Florida Department of Environmental Protection Florida Department of Agriculture and Consumer Services U.S. Army Corps of Engineers U.S. Fish and Wildlife Service Osceola County June 2011 Acknowledgments The South Florida Water Management District (SFWMD) would like to acknowledge the partner agencies and local governments and their staffs for their participation and commitment to the Kissimmee Chain of Lakes Long-Term Management Plan project. This dedicated team of professionals has exercised due diligence in the compilation, preparation, and review of the materials presented within this document and has worked to define and embrace a common vision of health for the Kissimmee Chain of Lakes resource. The SFWMD also acknowledges all the other stakeholders who have provided valuable insights into and perspectives on the value of the resource and the need for federal, state, and local government agencies to work together to preserve and protect the natural resources of central Florida. For further information about this document, please contact: Chris Carlson South Florida Water Management District 3301 Gun Club Road West Palm Beach, FL 33406 Telephone: (561) 682-6143 Email: [email protected] Interagency Team Draft Revision 2011 CONTENTS ACRONYMS AND ABBREVIATIONS ...................................................................................... -

Your Guide to Eating Fish Caught in Florida
Fish Consumption Advisories are published periodically by the Your Guide State of Florida to alert consumers about the possibility of chemically contaminated fish in Florida waters. To Eating The advisories are meant to inform the public of potential health risks of specific fish species from specific Fish Caught water bodies. In Florida Florida Department of Health Prepared in cooperation with the Florida Department of Environmental Protection and Agriculture and Consumer Services, and the Florida Fish and Wildlife Conservation Commission 2016 Florida Fish Advisories • Table 1: Eating Guidelines for Fresh Water Fish from Florida Waters page 1-33 • Table 2: Eating Guidelines for Marine and Estuarine Fish from Florida Waters page 34-35 • Table 3: Eating Guidelines for species from Florida Waters with Heavy Metals, Dioxin, Pesticides, Polychlorinated biphenyls (PCBs), or Saxitoxin Contamination page 36 Eating Fish is an important part of a healthy diet. Rich in vitamins and low in fat, fish contains protein we need for strong bodies. It is also an excellent source of nutrition for proper growth and development. In fact, the American Heart Association recommends that you eat two meals of fish or seafood every week. At the same time, most Florida seafood has low to medium levels of mercury. Depending on the age of the fish, the type of fish, and the condition of the water the fish lives in, the levels of mercury found in fish are different. While mercury in rivers, creeks, ponds, and lakes can build up in some fish to levels that can be harmful, most fish caught in Florida can be eaten without harm. -

2011 Everglade Snail Kite Nesting Summary
2011 Everglade Snail Kite nesting summary Troubling news in the Everglades region and Lake Okeechobee Kites suffered greatly in their critical habitats Lake Okeechobee and WCAs this year due to abnormally low water levels. There were 28 successful nests in Lake Okeechobee and the Everglades region in 2011. These areas are much larger than the Kissimmee Chain and are needed to sustain the long-term population viability of Kites, yet held far fewer nests. Photo Courtesy of Chuck Hanlon These shallow habitats are susceptible to short and long term impacts from droughts, like The Everglade Snail Kite (Kite), one of only three those in 2001, 2007, 2008, and now, 2011. Total System-Wide Performance Measures for the Lake Okeechobee’s littoral zone was bone dry in late Comprehensive Everglades Restoration Plan, has May 2011. Photo Courtesy of Paul Gray experienced an astonishing population decline in the past dozen years, decreasing from 3400 individuals to less than 700 today. Population models predict that if current trends continue, this iconic Florida species could become functionally extinct in a matter of 20 to 30 years. Because of the Kite’s low population, a successful Kite nesting season is crucial for the survival of the species. The 2011 nesting season for the Everglade Snail Kite had mixed results. Kites had a fairly successful year in the Kissimmee Chain of Lakes region. However, the news in the two most important nesting areas, Lake Okeechobee and the water conservation areas (WCAs) in the Everglades region, remains troubling. Table 1 depicts a preliminary assessment should take researchers until March 2012 to of the results, pending an official summary that complete. -

Lake Okeechobee
LAKE OKEECHOBEE: A Synthesis of Information and Recommendations for its Restoration Photo: NASA 2005 444 Brickell Ave, Suite 850 Miami, FL, 33131 305-371-6399 Cover Photo: Satellite Image of Lake Okeechobee Source: NASA To create an environmental ethic in all our citizens and to conserve and restore natural ecosystems, focusing on birds and other wildlife for the benefit of humanity and the earth’s biological diversity. Our special thanks to The Batchelor Foundation and The Everglades Foundation for their generous support of this important project For further information on this report, or for additional information regarding environmental restoration and conservation efforts in Florida, please contact us at: Florida State Office 444 Brickell Ave, Suite 850 Miami, FL, 33131 305-371-6399 305-371-6398 fax www.audubonofflorida.org This document should be cited as: Audubon of Florida. 2005. Lake Okeechobee: A synthesis of information and recommendations for its restoration. P. N. Gray, C. J. Farrell, M. L. Kraus, and A. H. Gromnicki, Eds., Audubon of Florida, Miami. 106 pp. + appendices i ACKNOWLEDGMENTS Documents that cover a broad range of topics require the authors to seek information from a very long list of people and institutions. Acknowledging all the help we received is not possible. Thus we necessarily restrict this list to certain people who contributed in significant ways. The SFWMD can be commended for their expansive research and monitoring programs around Lake Okeechobee. Dr. Karl E. Havens (now at the University of Florida) and Dr. Susan Gray of the SFWMD’s Lake Okeechobee Division have been indispensable in conducting research and disseminating results about the lake and its environment. -

Narcoossee Community Planning Process Is the Fulfillment of That Promise, and Was Initiated with the October 2010 Planning Process Kick-Off Workshop
Osceola County Neighborhood Draft Version 2; 21 - 22 June 2011 D r a f t table of contents i. vision + executive summary 1-1 ii. our foundation 2-1 iii. our future 3-1 iv. strategies for success 4-1 narcoossee community plan D r a f t acknowledgments Osceola County Citizens’ Advisory Committee Jeff Jones, AICP, Director of Strategic Initiatives Allen Doe, East Narcoossee Michelle Beamon, Project Manager Scott Sever, East Narcoossee Leigh Anne Wachter, AICP, Planner Mary Edgerton, North Narcoossee Jim Moore, North Narcoossee AECOM Design + Planning Thomas Atkins, South Narcoossee Betty Damke,South Narcoossee Bruce Meighen, AICP, Principal Sandy Siemon, Center Megan Moore, ASLA, Assoc. AIA, Project Manager Jewell Starling, Center Rebecca Brofft, Planner Cameron Gloss, AICP, Planner Aubrey Hake, Landscape Designer Joe McGrane, Landscape Architect Maria Michieli-Best, Quality Assurance Gregory Oakes, Landscape Designer Mackenzie Pett, Planner Scott Reyman,GIS narcoossee community plan D r a f t i . v i s i o n + executive summary narcoossee community plan D r a f t fulfilling a promise In June 2009, facing growth pressure from the north and east, the East Narcoossee Community and Osceola County conducted a ground-breaking planning process to collectively put forth their vision for what the community may become in the future. The three-day workshop facilitated that discussion and identified ways that the area can proactively plan for growth while preserving the community’s heritage and unique qualities. Residents were offered the chance to discuss these growth pressures, offer feedback on opportunities and values, identify an overall vision, work hand-in-hand with illustrators to develop concepts for integrating growth and development opportunities, and ultimately create an illustrative plan framework physically embodying this vision.