Streptophyte Algae and the Origin of Embryophytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Complete Plastome Sequences Of
Karol et al. BMC Evolutionary Biology 2010, 10:321 http://www.biomedcentral.com/1471-2148/10/321 RESEARCH ARTICLE Open Access Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages Kenneth G Karol1*, Kathiravetpillai Arumuganathan2, Jeffrey L Boore3,4, Aaron M Duffy5, Karin DE Everett6, John D Hall1, S Kellon Hansen5, Jennifer V Kuehl7, Dina F Mandoli6,8, Brent D Mishler9, Richard G Olmstead6, Karen S Renzaglia10, Paul G Wolf5 Abstract Background: Despite considerable progress in our understanding of land plant phylogeny, several nodes in the green tree of life remain poorly resolved. Furthermore, the bulk of currently available data come from only a subset of major land plant clades. Here we examine early land plant evolution using complete plastome sequences including two previously unexamined and phylogenetically critical lineages. To better understand the evolution of land plants and their plastomes, we examined aligned nucleotide sequences, indels, gene and nucleotide composition, inversions, and gene order at the boundaries of the inverted repeats. Results: We present the plastome sequences of Equisetum arvense, a horsetail, and of Isoetes flaccida,a heterosporous lycophyte. Phylogenetic analysis of aligned nucleotides from 49 plastome genes from 43 taxa supported monophyly for the following clades: embryophytes (land plants), lycophytes, monilophytes (leptosporangiate ferns + Angiopteris evecta + Psilotum nudum + Equisetum arvense), and seed plants. Resolution among the four monilophyte lineages remained moderate, although nucleotide analyses suggested that P. nudum and E. arvense form a clade sister to A. evecta + leptosporangiate ferns. Results from phylogenetic analyses of nucleotides were consistent with the distribution of plastome gene rearrangements and with analysis of sequence gaps resulting from insertions and deletions (indels). -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

(Polyphysacea, Dasycladales, Chlorophyta), Mid
Geologica Acta: an international earth science journal ISSN: 1695-6133 [email protected] Universitat de Barcelona España Granier, B.; Dias Brito, D.; Bucur, I. I. Clypeina tibanai, sp. nov. (Polyphysacea, Dasycladales, Chlorophyta), mid-Cretaceous green alga from the Potiguar Basin, Brazilian margin of the young South Atlantic Ocean Geologica Acta: an international earth science journal, vol. 12, núm. 3, julio-septiembre, 2014, pp. 227- 237 Universitat de Barcelona Barcelona, España Available in: http://www.redalyc.org/articulo.oa?id=50531655005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Geologica Acta, Vol.12, Nº 3, September 2014, 227-237 DOI: 10.1344/GeologicaActa2014.12.3.5 Clypeina tibanai, sp. nov. (Polyphysacea, Dasycladales, Chlorophyta), mid-Cretaceous green alga from the Potiguar Basin, Brazilian margin of the young South Atlantic Ocean B. GRANIER1 D. DIAS-BRITO2 I.I. BUCUR3 1Department of Ecology and Evolutionary Biology, The University of Kansas 1200 Sunnyside Avenue, Lawrence, Kansas 66045 (USA). E-mail: [email protected] 2UNESP - Universidade Estadual Paulista, Center for Geosciences Applied to Petroleum (UNESPetro) and Departamento de Geologia Caixa Postal 178, Av. 24 A, n º 1515, Bela Vista, CEP13506-900 - Rio Claro -SP (Brazil) 3Babeş-Bolyai University, Department of Geology and Center for Integrated Geological Studies Str. M. Kogalniceanu nr.1, 400084 Cluj-Napoca (Romania) ABS TRACT The fossil genus Clypeina (Michelin, 1845) comprises some 40 species. We describe Clypeina tibanai, a new spe- cies from ? upper Albian–Cenomanian strata of the Potiguar Basin, Brazil, characterised by closely set verticils of tubular, bended laterals. -

Botany Without Bias
editorial Botany without bias In the Gospel According to Matthew Chapter seven, Verse fve, Jesus says “frst cast out the beam out of thine own eye; and then shalt thou see clearly to cast out the mote out of thy brother’s eye”. We should remember this entreaty before too casually casting accusations of ‘plant blindness’. anguage usage helps maintain unconscious biases. In plant biology Lfor example, there is the careless use of the term ‘higher plants’, without thinking about its meaning or implication. If there is a definition of ‘higher plants’ then it is synonymous with vascular plants, but the image it conjures is of upstanding, leafy land-dwelling plants. The problem is that ‘higher’ is a charged term implying superiority of this group over their non-vascular cousins. This stratification is a manifestation of orthogenesis, the idea that evolution has both a direction and a goal. A perfect illustration of orthogenesis is the frequent meme of The Road to Homo Sapiens, the original version of which was drawn by Rudolph Zallinger for a 1965 edition of Life Nature Library1. Also known as The March of Progress, it shows a line of assumed human ancestors, starting with a gibbon-like in their News and Views3, “innovations spend much of their discussions on what Pliopithecus, processing from left to right, associated with improving water use the similarities of these organisms to becoming taller and more upright in stance, efficiency […] may be more fundamental angiosperms can tell about the history and culminating in a modern human. to the evolution of vascular plants than the of plants’ colonization of dry land, and The implication is clear, our evolutionary vascular system from which they derive much less on their characteristics and ancestors are only of interest as waypoints their name”. -

Mannitol Biosynthesis in Algae : More Widespread and Diverse Than Previously Thought
This is a repository copy of Mannitol biosynthesis in algae : more widespread and diverse than previously thought. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/113250/ Version: Accepted Version Article: Tonon, Thierry orcid.org/0000-0002-1454-6018, McQueen Mason, Simon John orcid.org/0000-0002-6781-4768 and Li, Yi (2017) Mannitol biosynthesis in algae : more widespread and diverse than previously thought. New Phytologist. pp. 1573-1579. ISSN 1469-8137 https://doi.org/10.1111/nph.14358 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ 1 Mannitol biosynthesis in algae: more widespread and diverse than previously thought. Thierry Tonon1,*, Yi Li1 and Simon McQueen-Mason1 1 Department of Biology, Centre for Novel Agricultural Products, University of York, Heslington, York, YO10 5DD, UK. * Author for correspondence: tel +44 1904328785; email [email protected] Key words: Algae, primary metabolism, mannitol biosynthesis, mannitol-1-phosphate dehydrogenase, mannitol-1-phosphatase, haloacid dehalogenase, histidine phosphatase, evolution of metabolic pathways. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
bioRxiv preprint doi: https://doi.org/10.1101/668475; this version posted June 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, Pavel Škaloudf, Charles F. Delwicheg, Andrew H. Knollh, John A. Raveni,j,k, Heroen Verbruggene, Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2 Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, Krijgslaan 281, 9000 Ghent, Belgium bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, Technologiepark 71, 9052 Zwijnaarde, Belgium cVIB Center for Plant Systems Biology, Technologiepark 71, 9052 Zwijnaarde, Belgium dBioinformatics Institute Ghent, Ghent University, Technologiepark 71, 9052 Zwijnaarde, Belgium eSchool of Biosciences, University of Melbourne, Melbourne, Victoria, Australia fDepartment of Botany, Faculty of Science, Charles University, Benátská 2, CZ-12800 Prague 2, Czech Republic gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742, USA hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, 02138, USA. iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee, DD2 5DA, UK jSchool of Biological Sciences, University of Western Australia (M048), 35 Stirling Highway, WA 6009, Australia kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia lMeise Botanic Garden, Nieuwelaan 38, 1860 Meise, Belgium 1To whom correspondence may be addressed. Email [email protected], [email protected], [email protected] or [email protected]. -
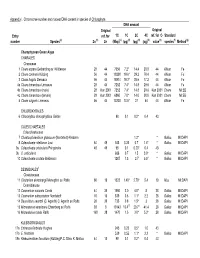
Green Appendix I
Appendix I. Chromsome number and nuclear DNA content in species of Chlorophyta DNA amount Original Original Entry ref. for 1C 1C 2C 4C ref. for C- Standard number Species(a) 2n (b) 2n (Mbp)(c) (pg)(d) (pg)(d) (pg)(d) value(e) species(f) Method(g) Charophycean Green Algae CHARALES Characeae 1 Chara aspera Detharding ex Willdenow 28 44 7056 7.2* 14.4 28.8 44 Allium Fe 2 Chara contraria Kützing 56 44 19208 19.6* 39.2 78.4 44 Allium Fe 3 Chara fragilis Desvaux 56 44 18914 19.3* 38.6 77.2 44 Allium Fe 4a Chara tomentosa Linnaeus 28 44 7252 7.4* 14.8 29.6 44 Allium Fe 4b Chara tomentosa (male) 28 Kun 2001 7252 7.4* 14.8 29.6 Kun 2001 Chara MI:EB 4c Chara tomentsoa (female) 28 Kun 2001 6860 7.0* 14.0 28.0 Kun 2001 Chara MI:EB 5 Chara vulgaris Linnaeus 56 44 13230 13.5* 27 54 44 Allium Fe CHLOROKYBALES 6 Chlorokybus atmosphyticus Geitler 98 0.1 0.2* 0.4 43 COLEOCHAETALES Coleochaetaceae 7 Chaetosphaeridium globosum (Nordstedt) Klebahn 1.2* * Gallus MI:DAPI 8 Coleochaete nitellarum Jost 84 49 343 0.35 0.7 1.4* * Gallus MI:DAPI 9a Coleochaete orbicularis Pringsheim 48 49 98 0.1 0.20* 0.4 43 9b C. orbicularis 686 0.7 1.5 3.0* * Gallus MI:DAPI 10 Coleochaete scutata Brébisson 1287 1.3 2.7 5.5* * Gallus MI:DAPI DESMIDIALES1 Closteriaceae 11 Closterium ehrenbergii Meneghini ex Ralfs 60 19 1323 1.40* 2.70* 5.4 19 Mus MI:DAPI Desmidiaceae 12 Cosmarium cucumis Corda 44 39 1960 2.0 4.0* 8 28 Gallus MI:DAPI 13 Cosmarium subcostatum Nordstedt 10 16 539 0.6 1.1* 2.2 28 Gallus MI:DAPI 14 Desmidium swartzii (C. -

Acetabularia Acetabulum
Plant Cell Physiol. 48(1): 122–133 (2007) doi:10.1093/pcp/pcl053, available online at www.pcp.oxfordjournals.org ß The Author 2006. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: [email protected] Spectroscopic and Biochemical Analysis of Regions of the Cell Wall of the Unicellular ‘Mannan Weed’, Acetabularia acetabulum Erin K. Dunn 1, 5, Douglas A. Shoue 2, 5, Xuemei Huang 3, Raymond E. Kline 4, Alex L. MacKay 3, Nicholas C. Carpita 2, Iain E.P. Taylor 4 and Dina F. Mandoli 1,Ã 1 Department of Biology, Center for Developmental Biology & Institute for Stem Cell and Regenerative Medicine, Box 35325, University of Washington, Seattle, WA 98195-5325, USA 2 Department of Botany and Plant Pathology, Purdue University, W. Lafayette, Indiana 47907-2054, USA 3 Department of Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada V6T 1Z1 4 Department of Botany, University of British Columbia, Vancouver, BC, Canada V6T 1Z4 Downloaded from https://academic.oup.com/pcp/article/48/1/122/2469303 by guest on 29 September 2021 Although the Dasycladalean alga Acetabularia acetabulum Introduction has long been known to contain mannan-rich walls, it is not known to what extent wall composition varies as a function of The Mediterranean coralline alga Acetabularia the elaborate cellular differentiation of this cell, nor has it acetabulum (previously classified as A. mediterranea)isa been determined what other polysaccharides -

Genomic and Fossil Windows Into the Secret Lives of the Most Ancient Fungi
REVIEWS Genomic and fossil windows into the secret lives of the most ancient fungi Mary L. Berbee 1 ✉ , Christine Strullu- Derrien 2,3, Pierre- Marc Delaux 4, Paul K. Strother5, Paul Kenrick 2, Marc- André Selosse 3,6 and John W. Taylor 7 Abstract | Fungi have crucial roles in modern ecosystems as decomposers and pathogens, and they engage in various mutualistic associations with other organisms, especially plants. They have a lengthy geological history, and there is an emerging understanding of their impact on the evolution of Earth systems on a large scale. In this Review, we focus on the roles of fungi in the establishment and early evolution of land and freshwater ecosystems. Today, questions of evolution over deep time are informed by discoveries of new fossils and evolutionary analysis of new genomes. Inferences can be drawn from evolutionary analysis by comparing the genes and genomes of fungi with the biochemistry and development of their plant and algal hosts. We then contrast this emerging picture against evidence from the fossil record to develop a new, integrated perspective on the origin and early evolution of fungi. Fungi are crucial for thriving land plant communities as enzymes surely kept pace3,16,17. Comparative analy- mycorrhizal symbionts1,2 and decomposers3. For perhaps sis of genomes of land plants18 and their closest algal 500 million years4,5, fungi have been supplying land plants relatives19,20 reveals the evolutionary origins of constitu- with phosphorus and other minerals, thereby speeding ents of plant immune systems that are fundamental both up photosynthesis and contributing to the drawdown for defence against fungal parasites and for the evolution 6–8 mycorrhizae21 1Department of Botany, of atmospheric carbon dioxide . -

Plant Evolution and Diversity B. Importance of Plants C. Where Do Plants Fit, Evolutionarily? What Are the Defining Traits of Pl
Plant Evolution and Diversity Reading: Chap. 30 A. Plants: fundamentals I. What is a plant? What does it do? A. Basic structure and function B. Why are plants important? - Photosynthesize C. What are plants, evolutionarily? -CO2 uptake D. Problems of living on land -O2 release II. Overview of major plant taxa - Water loss A. Bryophytes (seedless, nonvascular) - Water and nutrient uptake B. Pterophytes (seedless, vascular) C. Gymnosperms (seeds, vascular) -Grow D. Angiosperms (seeds, vascular, and flowers+fruits) Where? Which directions? II. Major evolutionary trends - Reproduce A. Vascular tissue, leaves, & roots B. Fertilization without water: pollen C. Dispersal: from spores to bare seeds to seeds in fruits D. Life cycles Æ reduction of gametophyte, dominance of sporophyte Fig. 1.10, Raven et al. B. Importance of plants C. Where do plants fit, evolutionarily? 1. Food – agriculture, ecosystems 2. Habitat 3. Fuel and fiber 4. Medicines 5. Ecosystem services How are protists related to higher plants? Algae are eukaryotic photosynthetic organisms that are not plants. Relationship to the protists What are the defining traits of plants? - Multicellular, eukaryotic, photosynthetic autotrophs - Cell chemistry: - Chlorophyll a and b - Cell walls of cellulose (plus other polymers) - Starch as a storage polymer - Most similar to some Chlorophyta: Charophyceans Fig. 29.8 Points 1. Photosynthetic protists are spread throughout many groups. 2. Plants are most closely related to the green algae, in particular, to the Charophyceans. Coleochaete 3. -

"Phycology". In: Encyclopedia of Life Science
Phycology Introductory article Ralph A Lewin, University of California, La Jolla, California, USA Article Contents Michael A Borowitzka, Murdoch University, Perth, Australia . General Features . Uses The study of algae is generally called ‘phycology’, from the Greek word phykos meaning . Noxious Algae ‘seaweed’. Just what algae are is difficult to define, because they belong to many different . Classification and unrelated classes including both prokaryotic and eukaryotic representatives. Broadly . Evolution speaking, the algae comprise all, mainly aquatic, plants that can use light energy to fix carbon from atmospheric CO2 and evolve oxygen, but which are not specialized land doi: 10.1038/npg.els.0004234 plants like mosses, ferns, coniferous trees and flowering plants. This is a negative definition, but it serves its purpose. General Features Algae range in size from microscopic unicells less than 1 mm several species are also of economic importance. Some in diameter to kelps as long as 60 m. They can be found in kinds are consumed as food by humans. These include almost all aqueous or moist habitats; in marine and fresh- the red alga Porphyra (also known as nori or laver), an water environments they are the main photosynthetic or- important ingredient of Japanese foods such as sushi. ganisms. They are also common in soils, salt lakes and hot Other algae commonly eaten in the Orient are the brown springs, and some can grow in snow and on rocks and the algae Laminaria and Undaria and the green algae Caulerpa bark of trees. Most algae normally require light, but some and Monostroma. The new science of molecular biology species can also grow in the dark if a suitable organic carbon has depended largely on the use of algal polysaccharides, source is available for nutrition.