(AMMODRAMUS NELSONI) SPARROWS by Jennifer Walsh University of New Hampshire, September 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grasshopper Sparrow,Ammodramus Savannarum Pratensis
COSEWIC Assessment and Status Report on the Grasshopper Sparrow Ammodramus savannarum pratensis pratensis subspecies (Ammodramus savannarum pratensis) in Canada SPECIAL CONCERN 2013 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2013. COSEWIC assessment and status report on the Grasshopper Sparrow pratensis subspecies Ammodramus savannarum pratensis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 36 pp. (www.registrelep- sararegistry.gc.ca/default_e.cfm). Production note: COSEWIC acknowledges Carl Savignac for writing the status report on the Grasshopper Sparrow pratensis subspecies, Ammodramus savannarum pratensis in Canada, prepared with the financial support of Environment Canada. This report was overseen and edited by Marty Leonard, Co-chair of the COSEWIC Birds Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bruant sauterelle de la sous- espèce de l’Est (Ammodramus savannarum pratensis) au Canada. Cover illustration/photo: Grasshopper Sparrow pratensis subspecies — photo by Jacques Bouvier. Her Majesty the Queen in Right of Canada, 2014. Catalogue No. CW69-14/681-2014E-PDF ISBN 978-1-100-23548-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2013 Common name Grasshopper Sparrow - pratensis subspecies Scientific name Ammodramus savannarum pratensis Status Special Concern Reason for designation In Canada, this grassland bird is restricted to southern Ontario and southwestern Quebec. -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -

Ammodramus Bairdii): a Technical Conservation Assessment
Baird’s Sparrow (Ammodramus bairdii): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project June 9, 2006 David A. Wiggins, Ph.D. Strix Ecological Research 1515 Classen Drive Oklahoma City, OK 73106 Peer Review Administered by Society for Conservation Biology Wiggins, D.A. (2006, June 9). Baird’s Sparrow (Ammodramus bairdii): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/ bairdssparrow.pdf [date of access]. ACKNOWLEDGMENTS Brenda Dale, Stephen Davis, Michael Green, and Stephanie Jones provided reprints and unpublished information on Baird’s sparrows – this assessment would not have been possible without their previous research work and helpful assistance. Greg Hayward and Gary Patton gave many useful tips for enhancing the structure and quality of this assessment. I also thank Rick Baydack, Scott Dieni, and Stephanie Jones for providing thorough reviews that greatly improved the quality of the assessment. AUTHOR’S BIOGRAPHY David Wiggins developed an early interest in ornithology. During his high school years, he worked as a museum assistant under Gary Schnell and George Sutton at the University of Oklahoma. He later earned degrees from the University of Oklahoma (B.Sc. in Zoology), Brock University (M.Sc. - Parental care in Common Terns, under the supervision of Ralph Morris), and Simon Fraser University (Ph.D. - Selection on life history traits in Tree Swallows, under the supervision of Nico Verbeek). This was followed by a National Science Foundation Post-doctoral fellowship at Uppsala University in Sweden, where he studied life history evolution in Collared Flycatchers, and later a Fulbright Fellowship working on the reproductive ecology of tits (Paridae) in Namibia and Zimbabwe. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Wildland Fire in Ecosystems: Effects of Fire on Fauna
United States Department of Agriculture Wildland Fire in Forest Service Rocky Mountain Ecosystems Research Station General Technical Report RMRS-GTR-42- volume 1 Effects of Fire on Fauna January 2000 Abstract _____________________________________ Smith, Jane Kapler, ed. 2000. Wildland fire in ecosystems: effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 83 p. Fires affect animals mainly through effects on their habitat. Fires often cause short-term increases in wildlife foods that contribute to increases in populations of some animals. These increases are moderated by the animals’ ability to thrive in the altered, often simplified, structure of the postfire environment. The extent of fire effects on animal communities generally depends on the extent of change in habitat structure and species composition caused by fire. Stand-replacement fires usually cause greater changes in the faunal communities of forests than in those of grasslands. Within forests, stand- replacement fires usually alter the animal community more dramatically than understory fires. Animal species are adapted to survive the pattern of fire frequency, season, size, severity, and uniformity that characterized their habitat in presettlement times. When fire frequency increases or decreases substantially or fire severity changes from presettlement patterns, habitat for many animal species declines. Keywords: fire effects, fire management, fire regime, habitat, succession, wildlife The volumes in “The Rainbow Series” will be published during the year 2000. To order, check the box or boxes below, fill in the address form, and send to the mailing address listed below. -

Species Assessment for Baird's Sparrow (Ammodramus Bairdii) in Wyoming
SPECIES ASSESSMENT FOR BAIRD ’S SPARROW (AMMODRAMUS BAIRDII ) IN WYOMING prepared by 1 2 ROBERT LUCE AND DOUG KEINATH 1 P.O. Box 2095, Sierra Vista, Arizona 85636, [email protected] 2 Zoology Program Manager, Wyoming Natural Diversity Database, University of Wyoming, 1000 E. University Ave, Dept. 3381, Laramie, Wyoming 82071; 307-766-3013; [email protected] prepared for United States Department of the Interior Bureau of Land Management Wyoming State Office Cheyenne, Wyoming December 2003 Luce and Keinath – Ammodramus bairdii December 2003 Table of Contents INTRODUCTION ................................................................................................................................. 5 NATURAL HISTORY ........................................................................................................................... 6 Morphological Description..................................................................................................... 6 Identification ...................................................................................................................................6 Vocalization ....................................................................................................................................7 Taxonomy and Distribution ................................................................................................... 7 Taxonomy .......................................................................................................................................7 Distribution -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -

5. WAGNER, SPARROW NEST DEFENSE, FFN 44(2).Pdf
Florida Field Naturalist 44(2):66-68, 2016. INSTANCES OF PARENTAL NEST DEFENSE BEHAVIOR IN A BACHMAN’S SPARROW (Peucaea aestivalis) AND A FLORIDA GRASSHOPPER SPARROW (Ammodramus savannarum floridanus) LINDSAY M. WAGNER1, STEPHEN N. HARRIS, AND ERIN L. HEWETT RAGHEB Fish and Wildlife Research Institute, Florida Fish and Wildlife Conservation Commission, 1105 Southwest Williston Road, Gainesville, Florida 32601 1E-mail: [email protected] Here we report instances of parental nest defense behavior in a Bachman’s Sparrow (Peucaea aestivalis) and a Florida Grasshopper Sparrow (Ammodramus savannarum floridanus) at Three Lakes Wildlife Management Area (TLWMA) in Osceola County, Florida. The Florida Grasshopper Sparrow is a critically endangered subspecies endemic to dry prairie habitat of south-central Florida (USFWS 1999). The Bachman’s Sparrow, also declining across much of its range (Sauer et al. 2011), is found throughout the southeastern United States in pine-dominated forests and a number of open habitats (Dunning 2006), including dry prairie (Perkins and Vickery 2005). We discovered and monitored nests of grassland birds as part of a multiyear study examining the role of prescribed fire and predation on reproductive success at TLWMA. In addition to traditional nest monitoring methods we installed motion-activated miniature nest cameras at the entrance of some nests (Cox et al. 2012). We reviewed the recordings from these cameras to identify nest predators, confirm nest fates, and document behaviors at the nest. During the 2015 breeding season at TLWMA we recorded 14 nest predation events, 13 of Florida Grasshopper Sparrow nests and 1 of a Bachman’s Sparrow nest. Of these 14 nests, 6 were preyed upon during daylight, and of those nests, we observed definite nest defense behaviors only at the two reported below. -
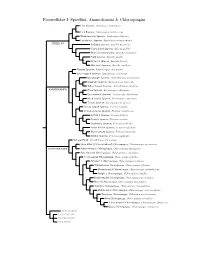
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Morphology Reveals the Unexpected Cryptic Diversity in Ceratophyllus Gallinae (Schrank, 1803) Infested Cyanistes Caeruleus Linnaeus, 1758 Nest Boxes
Acta Parasitologica (2020) 65:874–881 https://doi.org/10.1007/s11686-020-00239-6 ORIGINAL PAPER Morphology Reveals the Unexpected Cryptic Diversity in Ceratophyllus gallinae (Schrank, 1803) Infested Cyanistes caeruleus Linnaeus, 1758 Nest Boxes Olga Pawełczyk1 · Tomasz Postawa2 · Marian Blaski3 · Krzysztof Solarz1 Received: 6 June 2019 / Accepted: 29 May 2020 / Published online: 8 June 2020 © The Author(s) 2020 Abstract Purpose The main aim of our study was to examine morphological diferentiation between and within sex of hen feas— Ceratophyllus gallinae (Schrank, 1803) population collected from Eurasian blue tit (Cyanistes caeruleus Linnaeus, 1758), inhabiting nest boxes and to determine the morphological parameters diferentiating this population. Methods A total of 296 feas were collected (148 females and 148 males), determined to species and sex, then the following characters were measured in each of the examined feas: body length, body width, length of head, width of head, length of comb, height of comb, length of tarsus, length of thorax and length of abdomen. Results The comparison of body size showed the presence of two groups among female and male life forms of the hen fea, which mostly difered in length of abdomen, whereas the length of head and tarsus III were less variable. Conclusion Till now, the only certain information is the presence of two adult life forms of C. gallinae. The genesis of their creation is still unknown and we are not able to identify the mechanism responsible for the morphological diferentiation of feas collected from the same host. In order to fnd answer to this question, future research in the feld of molecular taxonomy is required. -

A Geometric Morphometric Analysis of Interspecific and Intersexual Size and Shape Variation in Colophon Haughtoni Barnard, 1929, and C
Org Divers Evol DOI 10.1007/s13127-016-0289-z ORIGINAL ARTICLE Does size matter for horny beetles? A geometric morphometric analysis of interspecific and intersexual size and shape variation in Colophon haughtoni Barnard, 1929, and C. kawaii Mizukami, 1997 (Coleoptera: Lucanidae) Thea Eldred1 & Carlo Meloro2 & Clarke Scholtz3 & Declan Murphy1 & Katie Fincken1 & Matt Hayward4 Received: 28 December 2015 /Accepted: 17 May 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Colophon is an understudied, rare and endangered biological and phylogenetic insights and enabling species stag beetle genus with all species endemic to isolated moun- identification. tain peaks in South Africa’s Western Cape. Geometric mor- phometrics was used to analyse intersexual and interspecific Keywords Colophon . Lucanidae . Geometric variation of size and shape in the mandibles, heads, pronota morphometrics . Morphology . Species identification . and elytra of two sympatric species: Colophon haughtoni and Sexual dimorphism Colophon kawaii. All measured structures showed significant sexual dimorphism, which may result from male-male com- petition for females. Female mandibles were too small and Introduction featureless for analysis, but male Colophon beetles possess large, ornate mandibles for fighting. Males had significantly Colophon Gray, 1832, is a genus of endangered montane stag larger heads and pronota that demonstrated shape changes beetles (subfamily Lucaninae Latreille, 1804 (Kim and Farrell which may relate to resource diversion to the mandibles and 2015)) endemic to the Western Cape Province of South Africa their supporting structures. Females are indistinguishable (Fig. 1) (Geertsema and Owen 2007; Switala et al. 2015). The across species, but males were accurately identified using first species was described by Gray (1832) in 1832, but over mandibles, heads and pronota.