Redalyc.GEOGRAPHIC BODY SIZE and SHAPE VARIATION in A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNIVERSIDAD DE GUAYAQUIL FACULTAD DE CIENCIAS NATURALES CARRERA DE BIOLOGÍA Trabajo De Titulación Previo a Obtener El Grado Ac
UNIVERSIDAD DE GUAYAQUIL FACULTAD DE CIENCIAS NATURALES CARRERA DE BIOLOGÍA Trabajo de titulación previo a obtener el grado académico de Biólogo Influencia del paisaje en el comportamiento territorial de la especie introducida Anolis sagrei (Duméril & Bibron, 1837) en ambientes urbanizados AUTOR: Jacob Agustín Guachisaca Salínas TUTOR: Blga. Andrea Narváez García, Ph.D. GUAYAQUIL, OCTUBRE, 2019 ANEXO 4 FACULTAD DE CIENCIAS NATURALES CARRERA DE BIOLOGÍA UNIDAD DE TITULACIÓN Guayaquil, 13 de agosto de 2019 Blga. Dialhy Coello, Mgs. DIRECTORA (e) DE LA CARRERA DE BIOLOGÍA FACULTAD DE CIENCIAS NATURALES UNIVERSIDAD DE GUAYAQUIL Ciudad. - De mis consideraciones: Envío a usted el informe correspondiente a la tutoría realizada al Trabajo de Titulación Influencia del paisaje en el comportamiento territorial de la especie introducida Anolis sagrei (Duméril & Bibron, 1837) en ambientes urbanizados del estudiante Jacob Agustín Guachisaca Salínas, indicando que ha cumplido con todos los parámetros establecidos en las normativas vigentes: El trabajo es el resultado de una investigación. El estudiante demuestra conocimiento profesional integral. El trabajo presenta una propuesta en el área de conocimiento. El nivel de argumentación es coherente con el campo de conocimiento. Adicionalmente, se adjunta el certificado del porcentaje de similitud y la valoración del trabajo de titulación con la respectiva calificación. Dando por concluida esta tutoría de trabajo de titulación, CERTIFICO, para los fines pertinentes, que el estudiante Jacob Agustín Guachisaca Salínas está apto para continuar el proceso de titulación de revisión final. Atentamente, _________________________ Andrea Narváez García, Ph.D. TUTOR DEL TRABAJO DE TITULACIÓN C.I. 1720145844 ANEXO 5 FACULTAD DE CIENCIAS NATURALES CARRERA DE BIOLOGÍA UNIDAD DE TITULACIÓN RÚBRICA DE EVALUACIÓN TRABAJO DE TITULACIÓN Título del Trabajo: Influencia del paisaje en el comportamiento territorial de la especie introducida Anolis sagrei (Duméril & Bibron, 1837) en ambientes urbanizados. -
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
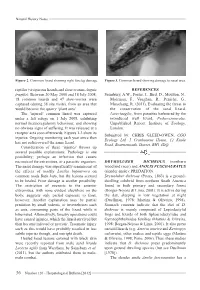
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

(2007) a Photographic Field Guide to the Reptiles and Amphibians Of
A Photographic Field Guide to the Reptiles and Amphibians of Dominica, West Indies Kristen Alexander Texas A&M University Dominica Study Abroad 2007 Dr. James Woolley Dr. Robert Wharton Abstract: A photographic reference is provided to the 21 reptiles and 4 amphibians reported from the island of Dominica. Descriptions and distribution data are provided for each species observed during this study. For those species that were not captured, a brief description compiled from various sources is included. Introduction: The island of Dominica is located in the Lesser Antilles and is one of the largest Eastern Caribbean islands at 45 km long and 16 km at its widest point (Malhotra and Thorpe, 1999). It is very mountainous which results in extremely varied distribution of habitats on the island ranging from elfin forest in the highest elevations, to rainforest in the mountains, to dry forest near the coast. The greatest density of reptiles is known to occur in these dry coastal areas (Evans and James, 1997). Dominica is home to 4 amphibian species and 21 (previously 20) reptile species. Five of these are endemic to the Lesser Antilles and 4 are endemic to the island of Dominica itself (Evans and James, 1997). The addition of Anolis cristatellus to species lists of Dominica has made many guides and species lists outdated. Evans and James (1997) provides a brief description of many of the species and their habitats, but this booklet is inadequate for easy, accurate identification. Previous student projects have documented the reptiles and amphibians of Dominica (Quick, 2001), but there is no good source for students to refer to for identification of these species. -

Checklist of Helminths from Lizards and Amphisbaenians (Reptilia, Squamata) of South America Ticle R A
The Journal of Venomous Animals and Toxins including Tropical Diseases ISSN 1678-9199 | 2010 | volume 16 | issue 4 | pages 543-572 Checklist of helminths from lizards and amphisbaenians (Reptilia, Squamata) of South America TICLE R A Ávila RW (1), Silva RJ (1) EVIEW R (1) Department of Parasitology, Botucatu Biosciences Institute, São Paulo State University (UNESP – Univ Estadual Paulista), Botucatu, São Paulo State, Brazil. Abstract: A comprehensive and up to date summary of the literature on the helminth parasites of lizards and amphisbaenians from South America is herein presented. One-hundred eighteen lizard species from twelve countries were reported in the literature harboring a total of 155 helminth species, being none acanthocephalans, 15 cestodes, 20 trematodes and 111 nematodes. Of these, one record was from Chile and French Guiana, three from Colombia, three from Uruguay, eight from Bolivia, nine from Surinam, 13 from Paraguay, 12 from Venezuela, 27 from Ecuador, 17 from Argentina, 39 from Peru and 103 from Brazil. The present list provides host, geographical distribution (with the respective biome, when possible), site of infection and references from the parasites. A systematic parasite-host list is also provided. Key words: Cestoda, Nematoda, Trematoda, Squamata, neotropical. INTRODUCTION The present checklist summarizes the diversity of helminths from lizards and amphisbaenians Parasitological studies on helminths that of South America, providing a host-parasite list infect squamates (particularly lizards) in South with localities and biomes. America had recent increased in the past few years, with many new records of hosts and/or STUDIED REGIONS localities and description of several new species (1-3). -

Anolis Aeneus (Bronze Anole)
UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour Anolis aeneus (Bronze Anole) Family: Polychrotidae (Anoles) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Bronze anole, Anolis aeneus. [http://www.trinidad-tobagoherps.org/Anolisaeneus.htm, downloaded 20 October 2012] TRAITS. Anolis aeneus can be distinguished by the blue-green ring around its eyes (Murphy 2011). The species is a medium sized anole, the length of males from the tip of the nose to the anus is 77 mm and females 55 mm (John et al. 2012). They have many lamellae (flaps) on their subdigital toepads. The dewlap (throat fan) extends from underneath their necks and has a pale gray, green or white colour, yellow or orange spots may also be present at the front edge of the dewlap (John et al. 2012). Colour: The dorsal side of the males may be grey, greyish brown, brown or a dull green, a bronze sheen is at times present, light or dark spots may be present in a crosswise pattern. The underside has a dull grey colour. The females may be grey or brown the mid-dorsal region can include a dark single stripe or a transverse stripe, juveniles are dark grey or brown (John et al. 2012). ECOLOGY. This species is endemic to Grenada and has been introduced to Trinidad and Tobago (Wikipedia 2012). It is an arboreal species and can therefore be found mostly on the trunk and branches of shaded trees, it is also populated in urban areas and can be observed on walls, railings and fences (Murphy 2011) it feeds on live insects and invertebrates such as crickets, roaches and spiders. -

Anolis Planiceps (Leaf Anole)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Anolis planiceps (Leaf Anole) Family: Polychrotidae (Anoles and Tree Lizards) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Leaf anole, Anolis planiceps. [http://www.trinidad-tobagoherps.org/Images/planiceps.jpg, downloaded 24 October 2016] TRAITS. Formerly known as Anolis chrysolepis or Norops chrysolepis, the leaf anole measures up to 76mm from snout to vent according (D'Angiolella et al., 2011). The pads of their feet are specialised to help them rest on leaves and trunks (Fig. 1). They have a spotted red patch of skin below theirs jaws, which is extendable, called the dewlap (Fig. 2). The region along the lizard's spine has larger scales than the adjacent areas with those located in the mid-dorsal area being the largest. Along their heads are two prominent ridges as well as ridged (keeled) scales located above the eyes (Fig. 3). The dorsal scales of the leaf anole are several shades of brown while the ventral scales are a pale cream colour; patterns vary greatly within populations (Fig. 4) (Vanzolini and Williams, 1970). Male anoles have longer tails and the females have wider bodies and smaller dewlaps than males (Vitt and Zani, 2011). DISTRIBUTION. Leaf anoles may be found in a relatively wide range from east Venezuela to Guyana, Suriname, Columbia, Trinidad and Brazil (Fig. 5). They are found throughout the island of Trinidad primarily in terrestrial, highly forested areas (D'Angiolella et al, 2011). UWI The Online Guide to the Animals of Trinidad and Tobago Diversity HABITAT AND ECOLOGY. -

Diversificação Morfológica E Molecular Em Lagartos Dactyloidae Sul-Americanos
MUSEU PARAENSE EMÍLIO GOELDI UNIVERSIDADE FEDERAL DO PARÁ PROGRAMA DE PÓS-GRADUAÇÃO EM ZOOLOGIA CURSO DE DOUTORADO EM ZOOLOGIA DIVERSIFICAÇÃO MORFOLÓGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS ANNELISE BATISTA D’ANGIOLELLA Belém - PA 2015 ANNELISE BATISTA D’ANGIOLELLA DIVERSIFICAÇÃO MORFOLOGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS Tese apresenta ao Programa de Pós-Graduação em Zoologia do convênio Universidade Federal do Pará e Museu Paraense Emílio Goeldi, para obtenção do título de doutora em zoologia. Orientadora: Dra. Tereza Cristina Ávila Pires Co-Orientadora: Dra. Ana Carolina Carnaval Belém - PA 2015 “É capaz quem pensa que é capaz.” ii Agradecimento Ao CNPq pela concessão da minha bolsa de pesquisa. A Capes pela Bolsa de Doutorado Sanduiche no exterior. À Teresa Avila-Pires, minha orientadora, por estar sempre disponível para ajudar, escutar e puxar a orelha! A minha co-orientadora Carol Carnaval, por ter me recebido de braços abertos em seu lab e por toda confiança e apoio. A Ana Prudente pelo passe livre à Coleção e sugestões dadas ao trabalho de hemipenis. Ao Tibério Burlamaqui por toda a ajuda com as análises moleculares e momentos de descontração! A todo o pessoal do laboratório de Herpetologia do MPEG pela companhia e troca de ideias, sempre ajudando quando possível. Ao lab de molecular que foi a minha casa nesses últimos quatro anos e a todos que por ele passaram e contribuíram de alguma forma com meu conhecimento, em especial a Áurea, Geraldo, e Joice. Aos meus filhos de quatro patas Pukey e Bingo por me amarem incondicionalmente. A dança, por ser meu refúgio e por não ter me deixado pirar! Ao meu amor, Bruno, por me inspirar diariamente a ser uma pessoa melhor! Por me impulsionar a ir além e por simplesmente existir em minha vida.. -

A Review of Sampling and Monitoring Methods for Beneficial Arthropods
insects Review A Review of Sampling and Monitoring Methods for Beneficial Arthropods in Agroecosystems Kenneth W. McCravy Department of Biological Sciences, Western Illinois University, 1 University Circle, Macomb, IL 61455, USA; [email protected]; Tel.: +1-309-298-2160 Received: 12 September 2018; Accepted: 19 November 2018; Published: 23 November 2018 Abstract: Beneficial arthropods provide many important ecosystem services. In agroecosystems, pollination and control of crop pests provide benefits worth billions of dollars annually. Effective sampling and monitoring of these beneficial arthropods is essential for ensuring their short- and long-term viability and effectiveness. There are numerous methods available for sampling beneficial arthropods in a variety of habitats, and these methods can vary in efficiency and effectiveness. In this paper I review active and passive sampling methods for non-Apis bees and arthropod natural enemies of agricultural pests, including methods for sampling flying insects, arthropods on vegetation and in soil and litter environments, and estimation of predation and parasitism rates. Sample sizes, lethal sampling, and the potential usefulness of bycatch are also discussed. Keywords: sampling methodology; bee monitoring; beneficial arthropods; natural enemy monitoring; vane traps; Malaise traps; bowl traps; pitfall traps; insect netting; epigeic arthropod sampling 1. Introduction To sustainably use the Earth’s resources for our benefit, it is essential that we understand the ecology of human-altered systems and the organisms that inhabit them. Agroecosystems include agricultural activities plus living and nonliving components that interact with these activities in a variety of ways. Beneficial arthropods, such as pollinators of crops and natural enemies of arthropod pests and weeds, play important roles in the economic and ecological success of agroecosystems. -

And Resurrection of Anolis (Diaphoranolis) Brooksi 1Steven Poe and 2Mason J
Ofcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 11(2) [General Section]: 1–16 (e141). urn:lsid:zoobank.org:pub:31FA8B4B-718B-4440-AE19-9E1AC95524BD Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi 1Steven Poe and 2Mason J. Ryan 1,3Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA 2Arizona Game and Fish Department, 5000 W. Carefree Highway, Phoenix, AZ 85086, USA Abstract.—The spectacular giant anole lizard Anolis insignis is widely distributed but infrequently collected outside of northern Costa Rica. We recently collected several individuals similar to Anolis insignis from localities in Panama and southern Costa Rica. These populations difer from type locality A. insignis in male dewlap color and morphology. We associate one set of these populations with Anolis (Diaphoranolis) brooksi Barbour from Darién, Panama, and describe two additional populations as new species. Keywords. Central America, Costa Rica, lizard, Panama, Reptilia, taxonomy Citation: Poe S and Ryan MJ. 2017. Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi. Amphibian & Reptile Conservation 11(2) [General Section]: 1–16 (e141). Copyright: © 2017 Poe and Ryan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International License, which permits unrestricted use for non-commercial and education purposes only, in any medium, provided the original author and the ofcial and authorized publication sources are recognized and properly credited. The ofcial and authorized publication credit sources, which will be duly enforced, are as follows: ofcial journal title Amphibian & Reptile Conservation; ofcial journal website <amphibian- reptile-conservation.org>. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

<I>ANOLIS</I> LIZARDS in the FOOD WEBS of STRUCTURALLY
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2016 ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA Nathan W. Turnbough University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Terrestrial and Aquatic Ecology Commons Recommended Citation Turnbough, Nathan W., "ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA. " PhD diss., University of Tennessee, 2016. https://trace.tennessee.edu/utk_graddiss/4174 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Nathan W. Turnbough entitled "ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Ecology and Evolutionary Biology. -

Anolis Cybotes Group)
Evolution, 57(10), 2003, pp. 2383±2397 PHYLOGENETIC ANALYSIS OF ECOLOGICAL AND MORPHOLOGICAL DIVERSIFICATION IN HISPANIOLAN TRUNK-GROUND ANOLES (ANOLIS CYBOTES GROUP) RICHARD E. GLOR,1,2 JASON J. KOLBE,1,3 ROBERT POWELL,4,5 ALLAN LARSON,1,6 AND JONATHAN B. LOSOS1,7 1Department of Biology, Campus Box 1137, Washington University, St. Louis, Missouri 63130-4899 2E-mail: [email protected] 3E-mail: [email protected] 4Department of Biology, Avila University, 11901 Wornall Road, Kansas City, Missouri 64145-1698 5E-mail: [email protected] 6E-mail: [email protected] 7E-mail: [email protected] Abstract. Anolis lizards in the Greater Antilles partition the structural microhabitats available at a given site into four to six distinct categories. Most microhabitat specialists, or ecomorphs, have evolved only once on each island, yet closely related species of the same ecomorph occur in different geographic macrohabitats across the island. The extent to which closely related species of the same ecomorph have diverged to adapt to different geographic macro- habitats is largely undocumented. On the island of Hispaniola, members of the Anolis cybotes species group belong to the trunk-ground ecomorph category. Despite evolutionary stability of their trunk-ground microhabitat, populations of the A. cybotes group have undergone an evolutionary radiation associated with geographically distinct macrohabitats. A combined phylogeographic and morphometric study of this group reveals a strong association between macrohabitat type and morphology independent of phylogeny. This association results from long-term morphological evolutionary stasis in populations associated with mesic-forest environments (A. c. cybotes and A. marcanoi) and predictable morphometric changes associated with entry into new macrohabitat types (i.e., xeric forests, high-altitude pine forest, rock outcrops).