Chronic Intestinal Ischemia: Measurement of the Total Splanchnic Blood Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gross Anatomical Studies on the Arterial Supply of the Intestinal Tract of the Goat
IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) e-ISSN: 2319-2380, p-ISSN: 2319-2372. Volume 10, Issue 1 Ver. I (January. 2017), PP 46-53 www.iosrjournals.org Gross Anatomical Studies on the Arterial Supply of the Intestinal Tract of the Goat Reda Mohamed1, 2*, ZeinAdam2 and Mohamed Gad2 1Department of Basic Veterinary Sciences, School of Veterinary Medicine, Faculty of Medical Sciences, University of the West Indies, Trinidad and Tobago. 2Anatomy and Embryology Department, Faculty of Veterinary Medicine, Beni Suef University Egypt. Abstract: The main purpose of this study was to convey a more precise explanation of the arterial supply of the intestinal tract of the goat. Fifteen adult healthy goats were used. Immediately after slaughtering of the goat, the thoracic part of the aorta (just prior to its passage through the hiatus aorticus of the diaphragm) was injected with gum milk latex (colored red) with carmine. The results showed that the duodenum was supplied by the cranial pancreaticoduodenal and caudal duodenal arteries. The jejunum was supplied by the jejunal arteries. The ileum was supplied by the ileal; mesenteric ileal and antimesenteric ileal arteries. The cecum was supplied by the cecal artery. The ascending colon was supplied by the colic branches and right colic arteries. The transverse colon was supplied by the middle colic artery. The descending colon was supplied by the middle and left colic arteries. The sigmoid colon was supplied by the sigmoid arteries. The rectum was supplied by the cranial; middle and caudal rectal arteries. Keywords: Anatomy,Arteries, Goat, Intestine I. Introduction Goats characterized by their high fertility rate and are of great economic value; being a cheap meat, milk and some industrial substances. -

PERIPHERAL VASCULATURE Average Vessel Diameter
PERIPHERAL VASCULATURE Average Vessel Diameter A Trio of Technologies. Peripheral Embolization Solutions A Single Solution. Fathom™ Steerable Guidewires Total Hypotube Tip Proximal/ UPN Length (cm) Length (cm) Length (cm) Distal O.D. Hepatic, Gastro-Intestinal and Splenic Vasculature 24 8-10 mm Common Iliac Artery 39 2-4 mm Internal Pudendal Artery M00150 900 0 140 10 10 cm .016 in 25 6-8 mm External Iliac Artery 40 2-4 mm Middle Rectal M00150 901 0 140 20 20 cm .016 in 26 4-6 mm Internal Iliac Artery 41 2-4 mm Obturator Artery M00150 910 0 180 10 10 cm .016 in 27 5-8 mm Renal Vein 42 2-4 mm Inferior Vesical Artery 28 43 M00150 911 0 180 20 20 cm .016 in 15-25 mm Vena Cava 2-4 mm Superficial Epigastric Artery 29 44 M00150 811 0 200 10 10 cm pre-shaped .014 in 6-8 mm Superior Mesenteric Artery 5-8 mm Femoral Artery 30 3-5 mm Inferior Mesenteric Artery 45 2-4 mm External Pudendal Artery M00150 810 0 200 10 10 cm .014 in 31 1-3 mm Intestinal Arteries M00150 814 0 300 10 10 cm .014 in 32 Male 2-4 mm Superior Rectal Artery A M00150 815 0 300 10 10 cm .014 in 33 1-3 mm Testicular Arteries 1-3 mm Middle Sacral Artery B 1-3 mm Testicular Veins 34 2-4 mm Inferior Epigastric Artery Direxion™ Torqueable Microcatheters 35 2-4 mm Iliolumbar Artery Female 36 2-4 mm Lateral Sacral Artery C 1-3 mm Ovarian Arteries Usable 37 D UPN Tip Shape RO Markers 3-5 mm Superior Gluteal Artery 1-3 mm Ovarian Veins Length (cm) 38 2-4 mm Inferior Gluteal Artery E 2-4 mm Uterine Artery M001195200 105 Straight 1 M001195210 130 Straight 1 M001195220 155 Straight 1 Pelvic -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Ascending Aorta to Intestinal Artery Bypass: Technical Aspects
EJVES Extra 9, 13–15 (2005) doi:10.1016/j.ejvsextra.2005.01.003, available online at http://www.sciencedirect.com on SHORT REPORT Ascending Aorta to Intestinal Artery Bypass: Technical Aspects L. Chiche* and E. Kieffer Department of Vascular Surgery, Pitie´-Salpeˆtrie`re University Hospital, Assistance Publique-Hoˆpitaux de Paris, Paris, France We describe the ascending aorta as an inflow in patients who need a mesenteric bypass and in whom the ascending aorta is the only remaining non-diseased segment. This operation was performed in five patients. Introduction interspace, is the preferred approach. Partial sterno- tomy can be extended by dividing the third costal A number of techniques can be used to treat chronic cartilage or by adding oblique sternotomy. Total intestinal ischemia.1,2 We use the ascending aorta as an sternotomy is necessary if access to the aortic arch or inflow in patients in whom the supraceliac, descend- supra-aortic trunks is required. ing thoracic aorta, the abdominal aorta or iliac arteries Median or subcostal laparotomy provides good are unsuitable due to the presence of extensive lesions access to intestinal artery lesions.1 The celiac trunk or previous surgery. The technique described here was (CT) can be exposed by the interhepatogastric route. performed in five patients (2.4%) out of 211 in whom Its origin is exposed after incision of the right crus of 309 intestinal artery revascularization procedures the diaphragm and division of the arcuate ligament. were carried out between 1990 and 2004. It is similar The superior mesenteric artery (SMA) can be to the classical technique of ascending aorta-abdomi- approached by the pre- or sub-duodenal intramesen- nal aorta bypass used in the management of thoraco- teric route or by a route between duodenum and 3 abdominal aortic lesions. -
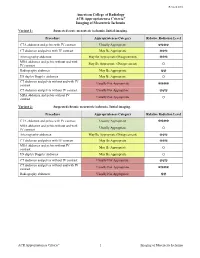
ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia Variant 1: Suspected acute mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ MRA abdomen and pelvis without and with May Be Appropriate (Disagreement) IV contrast O Radiography abdomen May Be Appropriate ☢☢ US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ MRA abdomen and pelvis without IV Usually Not Appropriate contrast O Variant 2: Suspected chronic mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ MRA abdomen and pelvis without and with Usually Appropriate IV contrast O Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ MRA abdomen and pelvis without IV May Be Appropriate contrast O US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ Radiography abdomen Usually Not Appropriate ☢☢ ACR Appropriateness Criteria® 1 Imaging of Mesenteric Ischemia IMAGING OF MESENTERIC ISCHEMIA Expert Panels on Vascular Imaging and Gastrointestinal Imaging: Michael Ginsburg, MDa; Piotr Obara, MDb; Drew L. Lambert, MDc; Michael Hanley, MDd; Michael L. Steigner, MDe; Marc A. Camacho, MD, MSf; Ankur Chandra, MDg; Kevin J. Chang, MDh; Kenneth L. -

Colonic Ischemia 9/21/14, 9:02 PM
Colonic ischemia 9/21/14, 9:02 PM Official reprint from UpToDate® www.uptodate.com ©2014 UpToDate® Colonic ischemia Authors Section Editors Deputy Editor Peter Grubel, MD John F Eidt, MD Kathryn A Collins, MD, PhD, FACS J Thomas Lamont, MD Joseph L Mills, Sr, MD Martin Weiser, MD All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Aug 2014. | This topic last updated: Aug 25, 2014. INTRODUCTION — Intestinal ischemia is caused by a reduction in blood flow, which can be related to acute arterial occlusion (embolic, thrombotic), venous thrombosis, or hypoperfusion of the mesenteric vasculature causing nonocclusive ischemia. Colonic ischemia is the most frequent form of intestinal ischemia, most often affecting the elderly [1]. Approximately 15 percent of patients with colonic ischemia develop gangrene, the consequences of which can be life-threatening, making rapid diagnosis and treatment imperative. The remainder develops nongangrenous ischemia, which is usually transient and resolves without sequelae [2]. However, some of these patients will have a more prolonged course or develop long-term complications, such as stricture or chronic ischemic colitis. The diagnosis and treatment of patients can be challenging since colonic ischemia often occurs in patients who are debilitated and have multiple medical problems. The clinical features, diagnosis, and treatment of ischemia affecting the colon and rectum will be reviewed here. Acute and chronic intestinal ischemia of the small intestine are discussed separately. (See "Acute mesenteric ischemia" and "Chronic mesenteric ischemia".) BLOOD SUPPLY OF THE COLON — The circulation to the large intestine and rectum is derived from the superior mesenteric artery (SMA), inferior mesenteric artery (IMA), and internal iliac arteries (figure 1). -

Mesenteric Ischemia
The new england journal of medicine Review Article Edward W. Campion, M.D., Editor Mesenteric Ischemia Daniel G. Clair, M.D., and Jocelyn M. Beach, M.D. esenteric ischemia is caused by blood flow that is insuffi- From the Cleveland Clinic Lerner College cient to meet the metabolic demands of the visceral organs. The severity of Medicine of Case Western Reserve University (D.G.C.) and the Department of ischemia and the type of organ involved depend on the affected vessel of Vascular Surgery, Heart and Vascular M Institute, Cleveland Clinic (D.G.C., J.M.B.) and the extent of collateral-vessel blood flow. Despite advances in the techniques used to treat problems in the mesenteric — both in Cleveland. Address reprint re- quests to Dr. Clair at the Department of circulation, the most critical factor influencing outcomes in patients with this Vascular Surgery, Cleveland Clinic, 9500 condition continues to be the speed of diagnosis and intervention. Although mes- Euclid Ave., Desk F30, Cleveland, OH enteric ischemia is an uncommon cause of abdominal pain, accounting for less 44195, or at claird@ ccf . org. than 1 of every 1000 hospital admissions, an inaccurate or delayed diagnosis can N Engl J Med 2016;374:959-68. result in catastrophic complications; mortality among patients in whom this con- DOI: 10.1056/NEJMra1503884 Copyright © 2016 Massachusetts Medical Society. dition is acute is 60 to 80%.1-3 This article highlights the pathophysiological features, diagnosis, and treat- ment of ischemic syndromes in the foregut and intestines. The goal of this review is to improve the understanding and management of this life-threatening disorder. -

Blood Vessels and Circulation
19 Blood Vessels and Circulation Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Functional Anatomy of Blood Vessels Learning Outcomes 19.1 Distinguish between the pulmonary and systemic circuits, and identify afferent and efferent blood vessels. 19.2 Distinguish among the types of blood vessels on the basis of their structure and function. 19.3 Describe the structures of capillaries and their functions in the exchange of dissolved materials between blood and interstitial fluid. 19.4 Describe the venous system, and indicate the distribution of blood within the cardiovascular system. © 2018 Pearson Education, Inc. Module 19.1: The heart pumps blood, in sequence, through the arteries, capillaries, and veins of the pulmonary and systemic circuits Blood vessels . Blood vessels conduct blood between the heart and peripheral tissues . Arteries (carry blood away from the heart) • Also called efferent vessels . Veins (carry blood to the heart) • Also called afferent vessels . Capillaries (exchange substances between blood and tissues) • Interconnect smallest arteries and smallest veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Two circuits 1. Pulmonary circuit • To and from gas exchange surfaces in the lungs 2. Systemic circuit • To and from rest of body © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits 1. Right atrium (entry chamber) • Collects blood from systemic circuit • To right ventricle to pulmonary circuit 2. Pulmonary circuit • Pulmonary arteries to pulmonary capillaries to pulmonary veins © 2018 Pearson Education, Inc. Module 19.1: Blood vessels and circuits Circulation pathway through circuits (continued) 3. Left atrium • Receives blood from pulmonary circuit • To left ventricle to systemic circuit 4. -

Acute Mesenteric Ischemia Revealing Cirrhosis: About a Clinical Case
Gastroenterology & Hepatology: Open Access Case Report Open Access Acute mesenteric ischemia revealing cirrhosis: about a clinical case Abstract Volume 11 Issue 2 - 2020 Acute mesenteric ischemia (AMI) is a rare and serious medical and surgical emergency, the 1 1 2 prognosis of which depends on the early diagnosis and appropriate treatment. It is caused Kpossou AR, Sokpon CNM, Doukpo MM, 3 4 5 1 by acute or chronic interruption of splanchno-mesenteric blood flow. This interruption may Gandji EW, Diallo K, Laleye C, Vignon RK, be due to embolism, thrombosis or intestinal hypoperfusion. We report a case of acute Eyisse-Kpossou YOT,5 Sehonou J1 mesenteric ischemia in a young subject in a National Hospital and University Center of 1Departement of Hepato-gastroenterology, National and Benin. He was a 23-year-old man with no history of admitting a diffuse abdominal pain, University Hospital Hubert Koutoukou Maga (CNHU-HKM), excruciating, sudden onset of torsion and associated vomiting and stopping of materials and Benin 2 gases. Emergency laparotomy revealed acute mesenteric ischemia with ileal necrosis. Ileal Departement of Hepato-gastroenterology, National and resection with endo-ileal endo- ileal anastomosis was performed. Etiological research has University Hospital Hubert Koutoukou Maga (CNHU-HKM), Benin revealed cirrhosis of undetermined cause. The postoperative course was marked by short 3Departement of Visceral Surgery, National and University bowel syndrome and ascitic decompensation of cirrhosis. Under symptomatic treatment, Hospital -

Diagnosis of Acute Abdominal Pain in Older Patients COREY LYON, LCDR, MC, USN, U.S
Diagnosis of Acute Abdominal Pain in Older Patients COREY LYON, LCDR, MC, USN, U.S. Naval Hospital Sigonella, Sigonella, Italy DWAYNE C. CLARK, M.D., Fond du Lac Regional Clinic, Fond du Lac, Wisconsin Acute abdominal pain is a common presenting complaint in older patients. Presentation may differ from that of the younger patient and is often complicated by coexistent disease, delays in presentation, and physical and social barri- ers. The physical examination can be misleadingly benign, even with catastrophic conditions such as abdominal aortic aneurysm rupture and mesenteric ischemia. Changes that occur in the biliary system because of aging make older patients vulnerable to acute cholecystitis, the most common indication for surgery in this population. In older patients with appendicitis, the initial diagnosis is correct only one half of the time, and there are increased rates of perforation and mortality when compared with younger patients. Medication use, gallstones, and alcohol use increase the risk of pancreatitis, and advanced age is an indicator of poor prognosis for this disease. Diverticulitis is a common cause of abdominal pain in the older patient; in appropriately selected patients, it may be treated on an outpatient basis with oral antibiotics. Small and large bowel obstructions, usually caused by adhesive disease or malignancy, are more common in the aged and often require surgery. Morbidity and mortality among older patients presenting with acute abdominal pain are high, and these patients often require hospitalization with prompt surgical consultation. (Am Fam Physician 2006;74:1537-44. Copyright © 2006 American Academy of Family Physicians.) cute abdominal pain (generally sultation.2,11 In retrospective studies, more defined as pain of less than one than one half of older patients presenting week’s duration) is a common to the emergency department with acute presenting complaint among abdominal pain required hospital admission, Aolder patients. -

Portal Vein Thrombosis
Portal Vein Thrombosis a a Syed Abdul Basit, MD , Christian D. Stone, MD, MPH , b, Robert Gish, MD * KEYWORDS Thrombosis Cirrhosis Portal vein Anticoagulation Thrombophilia Thromboelastography Malignancy KEY POINTS Portal vein thrombosis (PVT) is most commonly found in cirrhosis and often diagnosed incidentally by imaging studies. There are 3 important complications of PVT: Portal hypertension with gastrointestinal bleeding, small bowel ischemia, and acute ischemic hepatitis. Acute PVT is associated with symptoms of abdominal pain and/or acute ascites, and chronic PVT is characterized by the presence of collateral veins and risk of gastrointestinal bleeding. Treatment to prevent clot extension and possibly help to recanalize the portal vein is generally recommended for PVT in the absence of contraindications for anticoagulation. PVT may obviate liver transplantation owing to a lack of adequate vasculature for organ/ vessel anastomoses. INTRODUCTION Definition Portal vein thrombosis (PVT) is defined as a partial or complete occlusion of the lumen of the portal vein or its tributaries by thrombus formation. Diagnosis of PVT is occurring more frequently, oftentimes found incidentally, owing to the increasing use of abdom- inal imaging (Doppler ultrasonography, most commonly) performed in the course of routine patient evaluations and surveillance for liver cancer. There are 3 important clinical complications of PVT: Small bowel ischemia: PVT may extend hepatofugal, causing thrombosis of the mesenteric venous arch and resultant small intestinal ischemia, which has a mortality rate as high as 50% and may require small bowel or multivisceral trans- plant if the patient survives.1 a Section of Gastroenterology and Hepatology, University of Nevada School of Medicine, 2040 West Charleston Boulevard, Suite 300, Las Vegas, NV 89102, USA; b Division of Gastroenter- ology and Hepatology, Department of Medicine, Stanford University School of Medicine, Alway Building, Room M211, 300 Pasteur Drive, MC: 5187 Stanford, CA 94305-5187, USA * Corresponding author. -

Unusual Pancreatico-Mesenteric Vasculature: a Clinical Insight
Clinical Group Archives of Anatomy and Physiology DOI http://dx.doi.org/10.17352/aap.000001 ISSN: 2640-7957 CC By Shikha Singh, Jasbir Kaur, Jyoti Arora*, Renu Baliyan Jeph, Vandana Research Article Mehta and Rajesh Kumar Suri Unusual Pancreatico-Mesenteric Department of Anatomy, Vardhman Mahavir Medical College and Safdarjung Hospital, Ansari Nagar West, Delhi 110029, India Vasculature: A Clinical Insight Dates: Received: 09 November, 2016; Accepted: 03 December, 2016; Published: 06 December, 2016 *Corresponding author: Jyoti Arora, MBBS, MS, Abstract Professor, Department of Anatomy, Vardhman Mahavir Medical College and Safdarjung Hospital, Background: Awareness about the variable vascular anatomy of superior mesenteric artery is Ansari Nagar West, New Delhi, Delhi 110029, India, imperative for appropriate clinical management. Present study not only augments anatomical literature Tel: +91-99-99077775; Fax: +91-11-2375365; E-mail: pertaining to mesenteric vasculature but also adds to the clinical acumen of medical practitioners in their clinical endeavors. Keywords: Superior mesenteric artery; Anomalous Case summary: The present study reports the occurrence of anomalous branch, termed as branch; Inferior pancreatic artery; Inferior accessory inferior pancreatic artery stemming from superior mesenteric artery. Additionally inferior pancreaticoduodenal artery; Ventral splanchnic pancreaticoduodenal artery was seen to be dividing into right and left branches instead of usual anterior arteries and posterior branches. Right branch terminated