CPY Document
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identical Genomic Organization of Two Hemichordate Hox Clusters
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 22, 2053–2058, November 6, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.08.052 Report Identical Genomic Organization of Two Hemichordate Hox Clusters Robert Freeman,1,12 Tetsuro Ikuta,2,12 Michael Wu,3 [10] have similar organization to the hemichordate cluster, Ryo Koyanagi,2 Takeshi Kawashima,2 Kunifumi Tagawa,4 but with different posterior genes. These results provide Tom Humphreys,5 Guang-Chen Fang,6 Asao Fujiyama,7 genomic evidence for a well-ordered complex in the deutero- Hidetoshi Saiga,8 Christopher Lowe,9 Kim Worley,10 stome ancestor for the hox1–hox9/10 region, with the Jerry Jenkins,11 Jeremy Schmutz,11 Marc Kirschner,1 number and kind of posterior genes still to be elucidated. Daniel Rokhsar,3 Nori Satoh,2,* and John Gerhart3,* 1 Department of Systems Biology, Harvard Medical School, Results and Discussion Boston, MA 02114, USA 2 Marine Genomics Unit, Okinawa Institute of Science and Here we characterize the order, transcriptional orientation, and Technology Graduate University, Onna, clustering of the Hox genes of the genomes of two widely Okinawa 904-0495, Japan studied model hemichordates, Saccoglossus kowalevskii 3 Department of Molecular and Cell Biology, University of and Ptychodera flava [4, 11, 12], that represent two major California, Berkeley, Berkeley, CA 94720-3200, USA evolutionary branches of enteropneust hemichordates that 4 Marine Biological Laboratory, Graduate School of Science, diverged an estimated 400 million years ago (MYa) [13]: Hiroshima University, Onomichi, Hiroshima 722-0073, Japan Saccoglossus, of the direct developing harimaniids, and 5 Department of Cell and Molecular Biology, University of Ptychodera, of the indirect developing ptychoderids [14]. -

Platyhelminthes, Nemertea, and "Aschelminthes" - A
BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. III - Platyhelminthes, Nemertea, and "Aschelminthes" - A. Schmidt-Rhaesa PLATYHELMINTHES, NEMERTEA, AND “ASCHELMINTHES” A. Schmidt-Rhaesa University of Bielefeld, Germany Keywords: Platyhelminthes, Nemertea, Gnathifera, Gnathostomulida, Micrognathozoa, Rotifera, Acanthocephala, Cycliophora, Nemathelminthes, Gastrotricha, Nematoda, Nematomorpha, Priapulida, Kinorhyncha, Loricifera Contents 1. Introduction 2. General Morphology 3. Platyhelminthes, the Flatworms 4. Nemertea (Nemertini), the Ribbon Worms 5. “Aschelminthes” 5.1. Gnathifera 5.1.1. Gnathostomulida 5.1.2. Micrognathozoa (Limnognathia maerski) 5.1.3. Rotifera 5.1.4. Acanthocephala 5.1.5. Cycliophora (Symbion pandora) 5.2. Nemathelminthes 5.2.1. Gastrotricha 5.2.2. Nematoda, the Roundworms 5.2.3. Nematomorpha, the Horsehair Worms 5.2.4. Priapulida 5.2.5. Kinorhyncha 5.2.6. Loricifera Acknowledgements Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS This chapter provides information on several basal bilaterian groups: flatworms, nemerteans, Gnathifera,SAMPLE and Nemathelminthes. CHAPTERS These include species-rich taxa such as Nematoda and Platyhelminthes, and as taxa with few or even only one species, such as Micrognathozoa (Limnognathia maerski) and Cycliophora (Symbion pandora). All Acanthocephala and subgroups of Platyhelminthes and Nematoda, are parasites that often exhibit complex life cycles. Most of the taxa described are marine, but some have also invaded freshwater or the terrestrial environment. “Aschelminthes” are not a natural group, instead, two taxa have been recognized that were earlier summarized under this name. Gnathifera include taxa with a conspicuous jaw apparatus such as Gnathostomulida, Micrognathozoa, and Rotifera. Although they do not possess a jaw apparatus, Acanthocephala also belong to Gnathifera due to their epidermal structure. ©Encyclopedia of Life Support Systems (EOLSS) BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. -

Cannabis Dictionary
A MEDICAL DICTIONARY, BIBLIOGRAPHY, AND ANNOTATED RESEARCH GUIDE TO INTERNET REFERENCES JAMES N. PARKER, M.D. AND PHILIP M. PARKER, PH.D., EDITORS ii ICON Health Publications ICON Group International, Inc. 4370 La Jolla Village Drive, 4th Floor San Diego, CA 92122 USA Copyright 2003 by ICON Group International, Inc. Copyright 2003 by ICON Group International, Inc. All rights reserved. This book is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without written permission from the publisher. Printed in the United States of America. Last digit indicates print number: 10 9 8 7 6 4 5 3 2 1 Publisher, Health Care: Philip Parker, Ph.D. Editor(s): James Parker, M.D., Philip Parker, Ph.D. Publisher's note: The ideas, procedures, and suggestions contained in this book are not intended for the diagnosis or treatment of a health problem. As new medical or scientific information becomes available from academic and clinical research, recommended treatments and drug therapies may undergo changes. The authors, editors, and publisher have attempted to make the information in this book up to date and accurate in accord with accepted standards at the time of publication. The authors, editors, and publisher are not responsible for errors or omissions or for consequences from application of the book, and make no warranty, expressed or implied, in regard to the contents of this book. Any practice described in this book should be applied by the reader in accordance with professional standards of care used in regard to the unique circumstances that may apply in each situation. -

Ctenophore Relationships and Their Placement As the Sister Group to All Other Animals
ARTICLES DOI: 10.1038/s41559-017-0331-3 Ctenophore relationships and their placement as the sister group to all other animals Nathan V. Whelan 1,2*, Kevin M. Kocot3, Tatiana P. Moroz4, Krishanu Mukherjee4, Peter Williams4, Gustav Paulay5, Leonid L. Moroz 4,6* and Kenneth M. Halanych 1* Ctenophora, comprising approximately 200 described species, is an important lineage for understanding metazoan evolution and is of great ecological and economic importance. Ctenophore diversity includes species with unique colloblasts used for prey capture, smooth and striated muscles, benthic and pelagic lifestyles, and locomotion with ciliated paddles or muscular propul- sion. However, the ancestral states of traits are debated and relationships among many lineages are unresolved. Here, using 27 newly sequenced ctenophore transcriptomes, publicly available data and methods to control systematic error, we establish the placement of Ctenophora as the sister group to all other animals and refine the phylogenetic relationships within ctenophores. Molecular clock analyses suggest modern ctenophore diversity originated approximately 350 million years ago ± 88 million years, conflicting with previous hypotheses, which suggest it originated approximately 65 million years ago. We recover Euplokamis dunlapae—a species with striated muscles—as the sister lineage to other sampled ctenophores. Ancestral state reconstruction shows that the most recent common ancestor of extant ctenophores was pelagic, possessed tentacles, was bio- luminescent and did not have separate sexes. Our results imply at least two transitions from a pelagic to benthic lifestyle within Ctenophora, suggesting that such transitions were more common in animal diversification than previously thought. tenophores, or comb jellies, have successfully colonized from species across most of the known phylogenetic diversity of nearly every marine environment and can be key species in Ctenophora. -

Diagnosis and Differentiation of the Order Primates
YEARBOOK OF PHYSICAL ANTHROPOLOGY 30:75-105 (1987) Diagnosis and Differentiation of the Order Primates FREDERICK S. SZALAY, ALFRED L. ROSENBERGER, AND MARIAN DAGOSTO Department of Anthropolog* Hunter College, City University of New York, New York, New York 10021 (F.S.S.); University of Illinois, Urbanq Illinois 61801 (A.L. R.1; School of Medicine, Johns Hopkins University/ Baltimore, h4D 21218 (M.B.) KEY WORDS Semiorders Paromomyiformes and Euprimates, Suborders Strepsirhini and Haplorhini, Semisuborder Anthropoidea, Cranioskeletal morphology, Adapidae, Omomyidae, Grades vs. monophyletic (paraphyletic or holophyletic) taxa ABSTRACT We contrast our approach to a phylogenetic diagnosis of the order Primates, and its various supraspecific taxa, with definitional proce- dures. The order, which we divide into the semiorders Paromomyiformes and Euprimates, is clearly diagnosable on the basis of well-corroborated informa- tion from the fossil record. Lists of derived features which we hypothesize to have been fixed in the first representative species of the Primates, Eupri- mates, Strepsirhini, Haplorhini, and Anthropoidea, are presented. Our clas- sification of the order includes both holophyletic and paraphyletic groups, depending on the nature of the available evidence. We discuss in detail the problematic evidence of the basicranium in Paleo- gene primates and present new evidence for the resolution of previously controversial interpretations. We renew and expand our emphasis on postcra- nial analysis of fossil and living primates to show the importance of under- standing their evolutionary morphology and subsequent to this their use for understanding taxon phylogeny. We reject the much advocated %ladograms first, phylogeny next, and scenario third” approach which maintains that biologically founded character analysis, i.e., functional-adaptive analysis and paleontology, is irrelevant to genealogy hypotheses. -

A Revised Taxonomy of the Iguanodont Dinosaur Genera and Species
ARTICLE IN PRESS + MODEL Cretaceous Research xx (2007) 1e25 www.elsevier.com/locate/CretRes A revised taxonomy of the iguanodont dinosaur genera and species Gregory S. Paul 3109 North Calvert Station, Side Apartment, Baltimore, MD 21218-3807, USA Received 20 April 2006; accepted in revised form 27 April 2007 Abstract Criteria for designating dinosaur genera are inconsistent; some very similar species are highly split at the generic level, other anatomically disparate species are united at the same rank. Since the mid-1800s the classic genus Iguanodon has become a taxonomic grab-bag containing species spanning most of the Early Cretaceous of the northern hemisphere. Recently the genus was radically redesignated when the type was shifted from nondiagnostic English Valanginian teeth to a complete skull and skeleton of the heavily built, semi-quadrupedal I. bernissartensis from much younger Belgian sediments, even though the latter is very different in form from the gracile skeletal remains described by Mantell. Currently, iguanodont remains from Europe are usually assigned to either robust I. bernissartensis or gracile I. atherfieldensis, regardless of lo- cation or stage. A stratigraphic analysis is combined with a character census that shows the European iguanodonts are markedly more morpho- logically divergent than other dinosaur genera, and some appear phylogenetically more derived than others. Two new genera and a new species have been or are named for the gracile iguanodonts of the Wealden Supergroup; strongly bipedal Mantellisaurus atherfieldensis Paul (2006. Turning the old into the new: a separate genus for the gracile iguanodont from the Wealden of England. In: Carpenter, K. (Ed.), Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs. -

Development of the Annelid Axochord: Insights Into Notochord Evolution Antonella Lauri Et Al
RESEARCH | REPORTS ORIGIN OF NOTOCHORD by double WMISH (Fig. 2, F to L). Although none of the genes were exclusively expressed in the annelid mesodermal midline, their combined Development of the annelid coexpression was unique to these cells (implying that mesodermal midline in annelids and chor- damesoderm in vertebrates are more similar to axochord: Insights into each other than to any other tissue). It is unlikely that the molecular similarity between annelid notochord evolution and vertebrate mesodermal midline is due to in- dependent co-option of a conserved gene cas- Antonella Lauri,1*† Thibaut Brunet,1* Mette Handberg-Thorsager,1,2‡ sette, because this would require either that this Antje H.L. Fischer,1§ Oleg Simakov,1 Patrick R. H. Steinmetz,1‖ Raju Tomer,1,2¶ cassette was active elsewhere in the body (which Philipp J. Keller,2 Detlev Arendt1,3# is not the case) or that multiple identical inde- pendent events of co-option occurred (which is The origin of chordates has been debated for more than a century, with one key issue being unparsimonious). As in vertebrates, the meso- the emergence of the notochord. In vertebrates, the notochord develops by convergence dermal midline resembles the neuroectodermal and extension of the chordamesoderm, a population of midline cells of unique molecular midline, which expresses foxD, foxA, netrin, slit, identity. We identify a population of mesodermal cells in a developing invertebrate, the marine and noggin (figs. S6 and S7) but not brachyury or annelid Platynereis dumerilii, that converges and extends toward the midline and expresses a twist. However, unlike in chicken (10), the an- notochord-specific combination of genes. -

Defining Phyla: Evolutionary Pathways to Metazoan Body Plans
EVOLUTION & DEVELOPMENT 3:6, 432-442 (2001) Defining phyla: evolutionary pathways to metazoan body plans Allen G. Collins^ and James W. Valentine* Museum of Paleontology and Department of Integrative Biology, University of California, Berkeley, CA 94720, USA 'Author for correspondence (email: [email protected]) 'Present address: Section of Ecology, Befiavior, and Evolution, Division of Biology, University of California, San Diego, La Jolla, CA 92093-0116, USA SUMMARY Phyla are defined by two sets of criteria, one pothesis of Nielsen; the clonal hypothesis of Dewel; the set- morphological and the other historical. Molecular evidence aside cell hypothesis of Davidson et al.; and a benthic hy- permits the grouping of animals into clades and suggests that pothesis suggested by the fossil record. It is concluded that a some groups widely recognized as phyla are paraphyletic, benthic radiation of animals could have supplied the ances- while some may be polyphyletic; the phyletic status of crown tral lineages of all but a few phyla, is consistent with molecu- phyla is tabulated. Four recent evolutionary scenarios for the lar evidence, accords well with fossil evidence, and accounts origins of metazoan phyla and of supraphyletic clades are as- for some of the difficulties in phylogenetic analyses of phyla sessed in the light of a molecular phylogeny: the trochaea hy- based on morphological criteria. INTRODUCTION Molecules have provided an important operational ad- vance to addressing questions about the origins of animal Concepts of animal phyla have changed importantly from phyla. Molecular developmental and comparative genomic their origins in the six Linnaean classis and four Cuvieran evidence offer insights into the genetic bases of body plan embranchements. -

"Phoronida". In: Encyclopedia of Life Science
Phoronida Introductory article Christian C Emig, Centre d’Oce´anologie de Marseille CNRS, Marseille, France Article Contents . Basic Design The Phoronida, divided into two genera and 10 species, is a small marine group, which . Diversity and Lifestyles belongs to the phylum Lophophorata. Fossil History and Phylogeny Basic Design complexity of the lophophore from an oval to a helicoidal, The Phoronida is an exclusively marine group with a sessile through a horseshoe and spiral shape. This is related to an vermiform body enclosed in a tube (Figure 1) The body is increase in the number of tentacles, which is proportional composed of three distinct parts (prosome, mesosome and to the general body size. metasome), each containing its own coelomic cavity. The The metasome (or trunk) is slender and cylindrical, with prosome forms the epistome, a fold overhanging the mouth a bulb-like posterior end (or ampulla) that anchors the dorsally. The mesosome bears the lophophore, with the body in the rear end of the tube. It is separated from the rest mouth lying between its two rows of tentacles. The of the body by the diaphragm, a thick transverse septum lophophore is a terminal, bilaterally symmetrical, tentacle located behind the lophophore. crown, each tentacle having complex arrays of cilia for The digestive tract is U-shaped, bringing the anus close filter-feeding. Lophophore shape is a fairly constant to the mouth. The descending branch is divided into a short feature within each species, and there is an increasing oesophagus, followed by a long prestomach, then a stomach surrounded by a blood plexus. -

Radial Symmetry Or Bilateral Symmetry Or "Spherical Symmetry"
Symmetry in biology is the balanced distribution of duplicate body parts or shapes. The body plans of most multicellular organisms exhibit some form of symmetry, either radial symmetry or bilateral symmetry or "spherical symmetry". A small minority exhibit no symmetry (are asymmetric). In nature and biology, symmetry is approximate. For example, plant leaves, while considered symmetric, will rarely match up exactly when folded in half. Radial symmetry These organisms resemble a pie where several cutting planes produce roughly identical pieces. An organism with radial symmetry exhibits no left or right sides. They have a top and a bottom (dorsal and ventral surface) only. Animals Symmetry is important in the taxonomy of animals; animals with bilateral symmetry are classified in the taxon Bilateria, which is generally accepted to be a clade of the kingdom Animalia. Bilateral symmetry means capable of being split into two equal parts so that one part is a mirror image of the other. The line of symmetry lies dorso-ventrally and anterior-posteriorly. Most radially symmetric animals are symmetrical about an axis extending from the center of the oral surface, which contains the mouth, to the center of the opposite, or aboral, end. This type of symmetry is especially suitable for sessile animals such as the sea anemone, floating animals such as jellyfish, and slow moving organisms such as sea stars (see special forms of radial symmetry). Animals in the phyla cnidaria and echinodermata exhibit radial symmetry (although many sea anemones and some corals exhibit bilateral symmetry defined by a single structure, the siphonoglyph) (see Willmer, 1990). -

Flatworms/ Rotifers 1) Clade (Clades Are a Group of Related Phylum) Platyzoa A) Platyzoa Consist of 6 Phyla, However Most of T
Flatworms/ Rotifers 1) Clade (clades are a group of related phylum) Platyzoa a) Platyzoa consist of 6 phyla, however most of these phyla are represented by a very small number of species and are debated on where they fall taxonomically. b) These organisms represent the beginning or bilateral symmetry i) For organisms like sponges and Cnidarians it is to their advantage to be radial symmetrical because they can collect food from any angle ii) However now organisms show a distinct head and tail end and better movement to go after food items 2) Phylum Platyhelminthes a) This is the group of flat worms. b) It consist of four classes c) All but one class are parasitic in nature d) Feeding i) Platyhelminthes have an incomplete gut. ii) Most have a mouth, pharynx, and intestine (1) The advancement of having a small intestine increases surface area and thus increases the amount of nutrients absorbed. iii) Many of the non-parasitic species have a pharynx (connection between mouth and intestines) that has the ability to extend out of the mouth in order to gather resources. iv) Parasitic forms have to have some specialized feeding apparatus to extract nutrients from their host without causing too much harm. e) Sense organs i) Here is also an evolutionary advancement in nerve cells (1) The simplest forms are similar to Cnidarians, however, others have in addition one or more longitudinal nerve cords creating a “ladder” style pattern. (2) Towards the superior end there is a cluster of nerves that serves as a rudimentary brain ii) Tactile cells (cells that detect pressure) and chemoreceptors (cells that stimulate in response to a chemical) are abundant all over the body. -
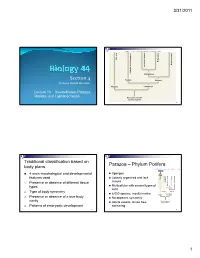
S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry