Algorithms for Biological Network Alignment A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
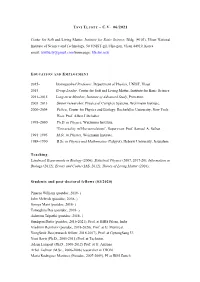
Tsvi Tlusty – C.V
TSVI TLUSTY – C.V. 06/2021 Center for Soft and Living Matter, Institute for Basic Science, Bldg. (#103), Ulsan National Institute of Science and Technology, 50 UNIST-gil, Ulju-gun, Ulsan 44919, Korea email: [email protected] homepage: life.ibs.re.kr EDUCATION AND EMPLOYMENT 2015– Distinguished Professor, Department of Physics, UNIST, Ulsan 2015– Group Leader, Center for Soft and Living Matter, Institute for Basic Science 2011–2015 Long-term Member, Institute of Advanced Study, Princeton. 2005–2013 Senior researcher, Physics of Complex Systems, Weizmann Institute. 2000–2004 Fellow, Center for Physics and Biology, Rockefeller University, New York. Host: Prof. Albert Libchaber 1995–2000 Ph.D. in Physics, Weizmann Institute, “Universality in Microemulsions”, Supervisor: Prof. Samuel A. Safran. 1991–1995 M.Sc. in Physics, Weizmann Institute. 1988–1990 B.Sc. in Physics and Mathematics (Talpyot), Hebrew University, Jerusalem. Teaching: Landmark Experiments in Biology (2006); Statistical Physics (2007, 2017-20); Information in Biology (2012); Errors and Codes (IAS, 2012); Theory of Living Matter (2016); Students and post-doctoral fellows (03/2020) Pineros William (postdoc, 2019- ) John Mcbride (postdoc, 2018- ) Somya Mani (postdoc, 2018- ) Tamoghna Das (postdoc, 2018- ) Ashwani Tripathi (postdoc, 2018- ) Sandipan Dutta (postdoc, 2016-2021), Prof. at BIRS Pileni, India Vladimir Reinharz (postdoc, 2018-2020), Prof. at U. Montreal. YongSeok Jho (research fellow, 2016-2017), Prof. at GyeongSang U. Yoni Savir (Ph.D., 2005-2011) Prof. at Technion. Adam Lampert (Ph.D., 2008-2012) Prof. at U. Arizona. Arbel Tadmor (M.Sc., 2006-2008) researcher at TRON. Maria Rodriguez Martinez (Postdoc, 2007-2009), PI at IBM Zurich Tamar Friedlander (Postdoc, 2009 -2012) Prof. -

Bonnie Berger Named ISCB 2019 ISCB Accomplishments by a Senior
F1000Research 2019, 8(ISCB Comm J):721 Last updated: 09 APR 2020 EDITORIAL Bonnie Berger named ISCB 2019 ISCB Accomplishments by a Senior Scientist Award recipient [version 1; peer review: not peer reviewed] Diane Kovats 1, Ron Shamir1,2, Christiana Fogg3 1International Society for Computational Biology, Leesburg, VA, USA 2Blavatnik School of Computer Science, Tel Aviv University, Tel Aviv, Israel 3Freelance Writer, Kensington, USA First published: 23 May 2019, 8(ISCB Comm J):721 ( Not Peer Reviewed v1 https://doi.org/10.12688/f1000research.19219.1) Latest published: 23 May 2019, 8(ISCB Comm J):721 ( This article is an Editorial and has not been subject https://doi.org/10.12688/f1000research.19219.1) to external peer review. Abstract Any comments on the article can be found at the The International Society for Computational Biology (ISCB) honors a leader in the fields of computational biology and bioinformatics each year with the end of the article. Accomplishments by a Senior Scientist Award. This award is the highest honor conferred by ISCB to a scientist who is recognized for significant research, education, and service contributions. Bonnie Berger, Simons Professor of Mathematics and Professor of Electrical Engineering and Computer Science at the Massachusetts Institute of Technology (MIT) is the 2019 recipient of the Accomplishments by a Senior Scientist Award. She is receiving her award and presenting a keynote address at the 2019 Joint International Conference on Intelligent Systems for Molecular Biology/European Conference on Computational Biology in Basel, Switzerland on July 21-25, 2019. Keywords ISCB, Bonnie Berger, Award This article is included in the International Society for Computational Biology Community Journal gateway. -

Classifying Transport Proteins Using Profile Hidden Markov Models And
Classifying Transport Proteins Using Profile Hidden Markov Models and Specificity Determining Sites Qing Ye A Thesis in The Department of Computer Science and Software Engineering Presented in Partial Fulfillment of the Requirements for the Degree of Master of Computer Science (MCompSc) at Concordia University Montréal, Québec, Canada April 2019 ⃝c Qing Ye, 2019 CONCORDIA UNIVERSITY School of Graduate Studies This is to certify that the thesis prepared By: Qing Ye Entitled: Classifying Transport Proteins Using Profile Hidden Markov Models and Specificity Determining Sites and submitted in partial fulfillment of the requirements for the degree of Master of Computer Science (MCompSc) complies with the regulations of this University and meets the accepted standards with respect to originality and quality. Signed by the Final Examining Committee: Chair Dr. T.-H. Chen Examiner Dr. T. Glatard Examiner Dr. A. Krzyzak Supervisor Dr. G. Butler Approved by Martin D. Pugh, Chair Department of Computer Science and Software Engineering 2019 Amir Asif, Dean Faculty of Engineering and Computer Science Abstract Classifying Transport Proteins Using Profile Hidden Markov Models and Specificity Determining Sites Qing Ye This thesis develops methods to classifiy the substrates transported across a membrane by a given transmembrane protein. Our methods use tools that predict specificity determining sites (SDS) after computing a multiple sequence alignment (MSA), and then building a profile Hidden Markov Model (HMM) using HMMER. In bioinformatics, HMMER is a set of widely used applications for sequence analysis based on profile HMM. Specificity determining sites (SDS) are the key positions in a protein sequence that play a crucial role in functional variation within the protein family during the course of evolution. -

A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast
A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast By Jessica M. Vera B.S. University of Wisconsin-Madison, 2007 A thesis submitted to the Faculty of the Graduate School in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Molecular, Cellular, and Developmental Biology 2015 This thesis entitled: A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast written by Jessica M. Vera has been approved for the Department of Molecular, Cellular, and Developmental Biology Tom Blumenthal Robin Dowell Date The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline iii Vera, Jessica M. (Ph.D., Molecular, Cellular and Developmental Biology) A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast Thesis Directed by Robin Dowell Cryptic unstable transcripts (CUTs) are a largely unexplored class of nuclear exosome degraded, non-coding RNAs in budding yeast. It is highly debated whether CUT transcription has a functional role in the cell or whether CUTs represent noise in the yeast transcriptome. I sought to ascertain the extent of conserved CUT expression across a variety of Saccharomyces yeast strains to further understand and characterize the nature of CUT expression. To this end I designed a Hidden Markov Model (HMM) to analyze strand-specific RNA sequencing data from nuclear exosome rrp6Δ mutants to identify and compare CUTs in four different yeast strains: S288c, Σ1278b, JAY291 (S.cerevisiae) and N17 (S.paradoxus). -

BIOGRAPHICAL SKETCH NAME: Berger
BIOGRAPHICAL SKETCH NAME: Berger, Bonnie eRA COMMONS USER NAME (credential, e.g., agency login): BABERGER POSITION TITLE: Simons Professor of Mathematics and Professor of Electrical Engineering and Computer Science EDUCATION/TRAINING (Begin with baccalaureate or other initial professional education, such as nursing, include postdoctoral training and residency training if applicable. Add/delete rows as necessary.) EDUCATION/TRAINING DEGREE Completion (if Date FIELD OF STUDY INSTITUTION AND LOCATION applicable) MM/YYYY Brandeis University, Waltham, MA AB 06/1983 Computer Science Massachusetts Institute of Technology SM 01/1986 Computer Science Massachusetts Institute of Technology Ph.D. 06/1990 Computer Science Massachusetts Institute of Technology Postdoc 06/1992 Applied Mathematics A. Personal Statement Advances in modern biology revolve around automated data collection and sharing of the large resulting datasets. I am considered a pioneer in the area of bringing computer algorithms to the study of biological data, and a founder in this community that I have witnessed grow so profoundly over the last 26 years. I have made major contributions to many areas of computational biology and biomedicine, largely, though not exclusively through algorithmic innovations, as demonstrated by nearly twenty thousand citations to my scientific papers and widely-used software. In recognition of my success, I have just been elected to the National Academy of Sciences and in 2019 received the ISCB Senior Scientist Award, the pinnacle award in computational biology. My research group works on diverse challenges, including Computational Genomics, High-throughput Technology Analysis and Design, Biological Networks, Structural Bioinformatics, Population Genetics and Biomedical Privacy. I spearheaded research on analyzing large and complex biological data sets through topological and machine learning approaches; e.g. -

Mechanistic Mathematical Modeling of Spatiotemporal Microtubule Dynamics and Regulation in Vivo
Research Collection Doctoral Thesis Mechanistic mathematical modeling of spatiotemporal microtubule dynamics and regulation in vivo Author(s): Widmer, Lukas A. Publication Date: 2018 Permanent Link: https://doi.org/10.3929/ethz-b-000328562 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library diss. eth no. 25588 MECHANISTICMATHEMATICAL MODELINGOFSPATIOTEMPORAL MICROTUBULEDYNAMICSAND REGULATION INVIVO A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH (dr. sc. eth zurich) presented by LUKASANDREASWIDMER msc. eth cbb born on 11. 03. 1987 citizen of luzern and ruswil lu, switzerland accepted on the recommendation of Prof. Dr. Jörg Stelling, examiner Prof. Dr. Yves Barral, co-examiner Prof. Dr. François Nédélec, co-examiner Prof. Dr. Linda Petzold, co-examiner 2018 Lukas Andreas Widmer Mechanistic mathematical modeling of spatiotemporal microtubule dynamics and regulation in vivo © 2018 ACKNOWLEDGEMENTS We are all much more than the sum of our work, and there is a great many whom I would like to thank for their support and encouragement, without which this thesis would not exist. I would like to thank my supervisor, Prof. Jörg Stelling, for giving me the opportunity to conduct my PhD research in his group. Jörg, you have been a great scientific mentor, and the scientific freedom you give your students is something I enjoyed a lot – you made it possible for me to develop my own theories, and put them to the test. I thank you for the trust you put into me, giving me a challenge to rise up to, and for always having an open door, whether in times of excitement or despair. -

DREAM: a Dialogue on Reverse Engineering Assessment And
DREAM:DREAM: aa DialogueDialogue onon ReverseReverse EngineeringEngineering AssessmentAssessment andand MethodsMethods Andrea Califano: MAGNet: Center for the Multiscale Analysis of Genetic and Cellular Networks C2B2: Center for Computational Biology and Bioinformatics ICRC: Irving Cancer RResearchesearch Center Columbia University 1 ReverseReverse EngineeringEngineering • Inference of a predictive (generative) model from data. E.g. argmax[P(Data|Model)] • Assumptions: – Model variables (E.g., DNA, mRNA, Proteins, cellular sub- structures) – Model variable space: At equilibrium, temporal dynamics, spatio- temporal dynamics, etc. – Model variable interactions: probabilistics (linear, non-linear), explicit kinetics, etc. – Model topology: known a-priori, inferred. • Question: – Model ~= Reality? ReverseReverse EngineeringEngineering Data Biological System Expression Proteomics > NFAT ATGATGGATG CTCGCATGAT CGACGATCAG GTGTAGCCTG High-throughput GGCTGGA Structure Sequence Biology … Biochemical Model Validation Control X-Y- Control X+Y+ Y X Z X+Y- X-Y+ Control Control Specific Prediction SomeSome ReverseReverse EngineeringEngineering MethodsMethods • Optimization: High-Dimensional objective function max corresponds to best topology – Liang S, Fuhrman S, Somogyi (REVEAL) – Gat-Viks and R. Shamir (Chain Functions) – Segal E, Shapira M, Regev A, Pe’er D, Botstein D, KolKollerler D, and Friedman N (Prob. Graphical Models) – Jing Yu, V. Anne Smith, Paul P. Wang, Alexander J. Hartemink, Erich D. Jarvis (Dynamic Bayesian Networks) – … • Regression: Create a general model of biochemical interactions and fit the parameters – Gardner TS, di Bernardo D, Lorentz D, and Collins JJ (NIR) – Alberto de la Fuente, Paul Brazhnik, Pedro Mendes – Roven C and Bussemaker H (REDUCE) – … • Probabilistic and Information Theoretic: Compute probability of interaction and filter with statistical criteria – Atul Butte et al. (Relevance Networks) – Gustavo Stolovitzky et al. (Co-Expression Networks) – Andrea CaCalifanolifano et al. -

Causal Queries from Observational Data in Biological Systems Via Bayesian Networks: an Empirical Study in Small Networks
Causal Queries from Observational Data in Biological Systems via Bayesian Networks: An Empirical Study in Small Networks Alex White and Matthieu Vignes Abstract Biological networks are a very convenient modelling and visualisation tool to discover knowledge from modern high-throughput genomics and post- genomics data sets. Indeed, biological entities are not isolated, but are components of complex multi-level systems. We go one step further and advocate for the con- sideration of causal representations of the interactions in living systems. We present the causal formalism and bring it out in the context of biological networks, when the data is observational. We also discuss its ability to decipher the causal infor- mation flow as observed in gene expression. We also illustrate our exploration by experiments on small simulated networks as well as on a real biological data set. Key words: Causal biological networks, Gene regulatory network reconstruction, Direct Acyclic Graph inference, Bayesian networks 1 Introduction Throughout their lifetime, organisms express their genetic program, i.e. the instruc- tion manual for molecular actions in every cell. The products of the expression of this program are messenger RNA (mRNA); the blueprints to produce proteins, the cornerstones of the living world. The diversity of shapes and the fate of cells is a re- sult of different readings of the genetic material, probably because of environmental factors, but also because of epigenetic organisational capacities. The genetic mate- rial appears regulated to produce what the organism needs in a specific situation. We now have access to rich genomics data sets. We see them as instantaneous images of cell activity from varied angles, through different filters. -

Multiscale Modeling in Systems Biology
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 2051 Multiscale Modeling in Systems Biology Methods and Perspectives ADRIEN COULIER ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-1225-5 UPPSALA URN urn:nbn:se:uu:diva-442412 2021 Dissertation presented at Uppsala University to be publicly examined in 2446 ITC, Lägerhyddsvägen 2, Uppsala, Friday, 10 September 2021 at 10:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Mark Chaplain (University of St Andrews). Abstract Coulier, A. 2021. Multiscale Modeling in Systems Biology. Methods and Perspectives. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 2051. 60 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-1225-5. In the last decades, mathematical and computational models have become ubiquitous to the field of systems biology. Specifically, the multiscale nature of biological processes makes the design and simulation of such models challenging. In this thesis we offer a perspective on available methods to study and simulate such models and how they can be combined to handle biological processes evolving at different scales. The contribution of this thesis is threefold. First, we introduce Orchestral, a multiscale modular framework to simulate multicellular models. By decoupling intracellular chemical kinetics, cell-cell signaling, and cellular mechanics by means of operator-splitting, it is able to combine existing software into one massively parallel simulation. Its modular structure makes it easy to replace its components, e.g. to adjust the level of modeling details. We demonstrate the scalability of our framework on both high performance clusters and in a cloud environment. -

EMBO Encounters Issue43.Pdf
WINTER 2019/2020 ISSUE 43 Nine group leaders selected Meet the first EMBO Global Investigators PAGE 6 Accelerating scientific publishing EMBO publishing costs Review Commons Making our journals’ platform announced finances public PAGE 3 PAGES 10 – 11 Welcome, Young Investigators! Contract replaces stipend Marking ten years 27 group leaders join the programme EMBO Postdoctoral Fellowships EMBO Molecular Medicine receive an update celebrates anniversary PAGES 4 – 5 PAGE 7 PAGE 13 www.embo.org TABLE OF CONTENTS EMBO NEWS EMBO news Review Commons: accelerating publishing Page 3 EMBO Molecular Medicine turns ten © Marietta Schupp, EMBL Photolab Marietta Schupp, © Page 13 Editorial MBO was founded by scientists for Introducing 27 new Young Investigators scientists. This philosophy remains at Pages 4-5 Ethe heart of our organization until today. EMBO Members are vital in the running of our Meet the first EMBO Global programmes and activities: they screen appli- Accelerating scientific publishing 17 journals on board Investigators cations, interview candidates, decide on fund- Review Commons will manage the transfer of ing, and provide strategic direction. On pages EMBO and ASAPbio announced pre-journal portable review platform the manuscript, reviews, and responses to affili- Page 6 8-9 four members describe why they chose to ate journals. A consortium of seventeen journals New members meet in Heidelberg dedicate their time to an EMBO Committee across six publishers (see box) have joined the Fellowships: from stipends to contracts Pages 14 – 15 and what they took away from the experience. n December 2019, EMBO, in partnership with decide to submit their work to a journal, it will project by committing to use the Review Commons Page 7 When EMBO was created, the focus lay ASAPbio, launched Review Commons, a multi- allow editors to make efficient editorial decisions referee reports for their independent editorial deci- specifically on fostering cross-border inter- Ipublisher partnership which aims to stream- based on existing referee comments. -

Assigning Folds to the Proteins Encoded by the Genome of Mycoplasma Genitalium (Protein Fold Recognition͞computer Analysis of Genome Sequences)
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 11929–11934, October 1997 Biophysics Assigning folds to the proteins encoded by the genome of Mycoplasma genitalium (protein fold recognitionycomputer analysis of genome sequences) DANIEL FISCHER* AND DAVID EISENBERG University of California, Los Angeles–Department of Energy Laboratory of Structural Biology and Molecular Medicine, Molecular Biology Institute, University of California, Los Angeles, Box 951570, Los Angeles, CA 90095-1570 Contributed by David Eisenberg, August 8, 1997 ABSTRACT A crucial step in exploiting the information genitalium (MG) (10), as a test of the capabilities of our inherent in genome sequences is to assign to each protein automatic fold recognition server and as a case study to sequence its three-dimensional fold and biological function. identify the difficulties facing automated fold assignment. Here we describe fold assignment for the proteins encoded by the small genome of Mycoplasma genitalium. The assignment MATERIALS AND METHODS was carried out by our computer server (http:yywww.doe- mbi.ucla.eduypeopleyfrsvryfrsvr.html), which assigns folds to The MG Sequences. The 468 MG sequences were obtained amino acid sequences by comparing sequence-derived predic- from The Institute for Genome Research (TIGR) through its tions with known structures. Of the total of 468 protein ORFs, Web address: http:yywww.tigr.orgytdbymdbymgdbymgd- 103 (22%) can be assigned a known protein fold with high b.html. Three types of annotation (based on searches in the confidence, as cross-validated with tests on known structures. sequence database) accompany each TIGR sequence (10): (i) Of these sequences, 75 (16%) show enough sequence similarity functional assignment—a clear sequence similarity with a to proteins of known structure that they can also be detected protein of known function from another organism was found by traditional sequence–sequence comparison methods. -

Research Report 2006 Max Planck Institute for Molecular Genetics, Berlin Imprint | Research Report 2006
MAX PLANCK INSTITUTE FOR MOLECULAR GENETICS Research Report 2006 Max Planck Institute for Molecular Genetics, Berlin Imprint | Research Report 2006 Published by the Max Planck Institute for Molecular Genetics (MPIMG), Berlin, Germany, August 2006 Editorial Board Bernhard Herrmann, Hans Lehrach, H.-Hilger Ropers, Martin Vingron Coordination Claudia Falter, Ingrid Stark Design & Production UNICOM Werbeagentur GmbH, Berlin Number of copies: 1,500 Photos Katrin Ullrich, MPIMG; David Ausserhofer Contact Max Planck Institute for Molecular Genetics Ihnestr. 63–73 14195 Berlin, Germany Phone: +49 (0)30-8413 - 0 Fax: +49 (0)30-8413 - 1207 Email: [email protected] For further information about the MPIMG please see our website: www.molgen.mpg.de MPI for Molecular Genetics Research Report 2006 Table of Contents The Max Planck Institute for Molecular Genetics . 4 • Organisational Structure. 4 • MPIMG – Mission, Development of the Institute, Research Concept. .5 Department of Developmental Genetics (Bernhard Herrmann) . 7 • Transmission ratio distortion (Hermann Bauer) . .11 • Signal Transduction in Embryogenesis and Tumor Progression (Markus Morkel). 14 • Development of Endodermal Organs (Heiner Schrewe) . 16 • Gene Expression and 3D-Reconstruction (Ralf Spörle). 18 • Somitogenesis (Lars Wittler). 21 Department of Vertebrate Genomics (Hans Lehrach) . 25 • Molecular Embryology and Aging (James Adjaye). .31 • Protein Expression and Protein Structure (Konrad Büssow). .34 • Mass Spectrometry (Johan Gobom). 37 • Bioinformatics (Ralf Herwig). .40 • Comparative and Functional Genomics (Heinz Himmelbauer). 44 • Genetic Variation (Margret Hoehe). 48 • Cell Arrays/Oligofingerprinting (Michal Janitz). .52 • Kinetic Modeling (Edda Klipp) . .56 • In Vitro Ligand Screening (Zoltán Konthur). .60 • Neurodegenerative Disorders (Sylvia Krobitsch). .64 • Protein Complexes & Cell Organelle Assembly/ USN (Bodo Lange/Thorsten Mielke). .67 • Automation & Technology Development (Hans Lehrach).