Nomenclature of the Perovskite Supergroup: a Hierarchical System of Classification Based on Crystal Structure and Composition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recycling of Hazardous Waste from Tertiary Aluminium Industry in A
CORE Metadata, citation and similar papers at core.ac.uk Provided by Digital.CSIC 1 Recycling of hazardous waste from tertiary aluminium 2 industry in a value-added material 3 4 Laura Gonzalo-Delgado1, Aurora López-Delgado1*, Félix Antonio López1, Francisco 5 José Alguacil1 and Sol López-Andrés2 6 7 1Nacional Centre for Metallurgical Research, CSIC. Avda. Gregorio del Amo, 8. 28040. 8 Madrid. Spain. 9 2Dpt. Crystallography and Mineralogy. Fac. of Geology. University Complutense of 10 Madrid. Spain. 11 *Corresponding author e-mail: [email protected] 12 13 Abstract 14 15 The recent European Directive on waste, 2008/98/EC seeks to reduce the 16 exploitation of natural resources through the use of secondary resource management. 17 Thus the main objective of this paper is to explore how a waste could cease to be 18 considered as waste and could be utilized for a specific purpose. In this way, a 19 hazardous waste from the tertiary aluminium industry was studied for its use as a raw 20 material in the synthesis of an added value product, boehmite. This waste is classified as 21 a hazardous residue, principally because in the presence of water or humidity, it releases 22 toxic gases such as hydrogen, ammonia, methane and hydrogen sulphide. The low 23 temperature hydrothermal method developed permits the recovery of 90% of the 24 aluminium content in the residue in the form of a high purity (96%) AlOOH (boehmite). 25 The method of synthesis consists of an initial HCl digestion followed by a gel 26 precipitation. In the first stage a 10% HCl solution is used to yield a 12.63 g.l-1 Al3+ 27 solution. -

The Solubility of Aluminium in Melts Containing Aluminium Halides
THE SOLUBILITY OF ALUMINIUM IN MELTS CONTAINING ALUMINIUM HALIDES A Thesis presented for the degree of Doctor of Philosophy in the University of London by John Dyson Usher London, May 1965 ABSTRACT A technique for measuring the solubility of aluminium in NaF-A1F3 and Nall-A1F3+5%A1203 melts has been developed using a refractory titanium boride- carbide crucible and a gas volumetric method of analysis. Experiments were conducted at 10209 1100 and 1180°C; in both systems no change in solubility limit was detected over the experimental composition range of 25.6 - 36.6 and 25.8 - 31.9 mol % AlF3 respectively. The figures in the pure fluoride are 0.022 0.041 and 0.072 wt %; in the alumina melts 0.073, 0.121 and 0.169 wt %. Solution mechanisms have been suggested which account for the observed behaviour, and an attempt has been made to interpret the current inefficiency process in the reduction cell in terms of the results. CONTENTS 1. INTRODUCTION Page 1.1 Aluminium Reduction Cell - Brief Description 1 1.2 Current Efficiency 3 1.2.1 Measurement 3 1.2.2 Cell Variables and Current Efficiency 5 1.2.2 Postulated Mechanisms of Metal Lose 6 1.3 Origins of Present Work 7 1.3.1 Information Required 7 1.3.A Preliminary Theoretical Approach 9 1.4 Metal-Molten Salt Solutions 11 1.4.1 General 11 1.4.2 Nature of Metal-Molten Salt Solutions 16 1.4.3 Extent of Solubility 19 1.4.4 Effect of Foreign Ions on Metal Solubility 23 1.5 Structure of Cryolite and Cryolite-Alumina Melts 25 1.5.1 Pure Molten Cryolite 25 1.5.2 Cryolite-Alumina Melts 33 1.6 Thermodynamics of Aluminium+Cryolite 40 1.6.1 Equilibrium 1.6(i): Experimental 40 1.6.2 Equilibrium 1.6(i): Calculations 42 1.6.3 Equilibrium 1.6(ii) 45 1.7 Previous Experimental Work 46 1.7.1 Analysis by Metal Weight Loss 48 1.7.2 Gas Volumetric Method 52 2. -

Recovery of Carbon and Cryolite from Spent Pot Lining of Aluminium Reduction Cells by Chemical Leaching
Trans. Nonferrous Met. Soc. China 22(2012) 222−227 Recovery of carbon and cryolite from spent pot lining of aluminium reduction cells by chemical leaching SHI Zhongning, LI Wei, HU Xianwei, REN Bijun, GAO Bingliang, WANG Zhaowen School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China Received 29 November 2010; accepted 8 October 2011 Abstract: A twostep alkalineacidic leaching process was conducted to separate the cryolite from spent pot lining and to purify the carbon. The influencing factors of temperature, time, and the ratio of liquid to solid in alkaline and acidic leaching were investigated. The results show that the recovery of soluble compounds of Na3AlF6 and Al2O3 dissolving into the solution during the NaOH leaching is 65.0%,and the purity of carbon reaches 72.7%. During the next step of HCl leaching, the recovery of soluble compounds of CaF2 and NaAl11O17 dissolving into the HCl solution is 96.2%, and the carbon purity increases to 96.4%. By mixing the acidic leaching solution and the alkaline leaching solution, the cryolite precipitates under a suitable conditions of pH value 9 at 70 °C for 2 h. The cryolite precipitating rate is 95.6%, and the purity of Na3AlF6 obtained is 96.4%. Key words: spent pot lining; recovery; chemical leaching; aluminium electrolysis method separates the carbon and fluoride according to 1 Introduction the difference in solubility, density and surface properties between carbon and fluorides. For example, the froth With a rapid development of the primary aluminium flotation technology is a typically physical separation industry, more and more spent pot lining (SPL) is process for SPL treatment [3]. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

New Mineral Names*,†
American Mineralogist, Volume 100, pages 1649–1654, 2015 New Mineral Names*,† DMITRIY I. BELAKOVSKIY1 AND OLIVIER C. GAGNE2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada IN THIS ISSUE This New Mineral Names has entries for 10 new minerals, including debattistiite, evdokimovite, ferdowsiite, karpovite, kolskyite, markhininite, protochabournéite, raberite, shulamitite, and vendidaite. DEBATTISTIITE* for 795 unique I > 2σ(I) reflections] corner-sharing As(S,Te)3 A. Guastoni, L. Bindi, and F. Nestola (2012) Debattistiite, pyramids form three-membered distorted rings linked by Ag atoms in triangular or distorted tetrahedral coordination. Certain Ag9Hg0.5As6S12Te2, a new Te-bearing sulfosalt from Len- genbach quarry, Binn valley, Switzerland: description and features of that linkage are similar to those in the structures of crystal structure. Mineralogical Magazine, 76(3), 743–750. trechmannite and minerals of pearceite–polybasite group. Of the seven anion positions, one is almost fully occupied by Te (Te0.93S0.07). The Hg atom is in a nearly perfect linear coordination Debattistiite (IMA 2011-098), ideally Ag9Hg0.5As6S12Te2, is a new mineral discovered in the famous for Pb-Cu-Ag-As-Tl with two Te/S atoms. One of five Ag sites and Hg site, which are bearing sulfosalts Lengenbach quarry in the Binn Valley, Valais, very close (separation 1.137 Å), are partially occupied (50%). Switzerland. Debattistiite has been identified in two specimens Thus there is a statistical distribution (50:50) between Hg(Te,S)2 from zone 1 of the quarry in cavities in dolomitic marble with and AgS2(Te,S)2 polyhedra in the structure. -

Al, Si Retained
Abstracts of Workshop on Transport Properties of the Lower Mantle, Yunishigawa-onsen, Tochigi-ken, Japan, 2008 Scale limits on free-silica seismic scatterers in the lower mantle Craig R. Bina Dept. of Earth and Planetary Sciences, Northwestern University, U.S.A. Seismic velocity anomalies and scatterers of seismic energy in the lower mantle often are attributed to subducted oceanic lithosphere. In particular, silica-saturated basalts in oceanic crust (MORB) under lower mantle conditions should contain high-pressure phases of free silica among assemblages otherwise dominated by silicate perovskite. Free silica phases such as stishovite are expected to generate seismic velocity anomalies that are fast by a few percent relative to surrounding ultramafic peridotite or harzburgite assemblages (Mattern et al. 2002, Bina 2003a, Ricard et al. 2005), and post-stishovite phases such as CaCl2-structured silica may also generate locally slow shear-wave velocity anomalies due to displacive shear-mode transitions (Bina 2003b, Lakshtanov 2007, Konishi et al. 2008). Such models, however, must address the thermodynamic instability of free silica phases in the presence of peridotites or harzburgites, as the silica will react with adjacent ferropericlase (magnesiowüstite) to form silicate perovskite. Thus, any free silica phases preserved in the lower mantle may persist as armored relics, in which silica phases are insulated from surrounding ferropericlase phases by coronas of silicate perovskite. This parallels the situation in crustal metamorphic rocks where, for example, staurolite crystals are often found as armored relics within garnet phases or spinel crystals can be found as relics armored by staurolite poikiloblasts (Whitney 1991, Gil Ibarguchi et al. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -

Brownmillerite Ca2(Al, Fe )2O5 C 2001-2005 Mineral Data Publishing, Version 1
3+ Brownmillerite Ca2(Al, Fe )2O5 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: mm2. As square platelets, to about 60 µm; massive. Physical Properties: Hardness = n.d. D(meas.) = 3.76 D(calc.) = 3.68–3.73 Optical Properties: Semitransparent. Color: Reddish brown. Optical Class: Biaxial (–). Pleochroism: Distinct; X = Y = yellow-brown; Z = dark brown. Orientation: Y and Z lie in the plane of the platelets; extinction in that plane is diagonal. α = < 2.02 β = > 2.02 γ = > 2.02 2V(meas.) = n.d. Cell Data: Space Group: Ibm2. a = 5.584(5) b = 14.60(1) c = 5.374(5) Z = 2 X-ray Powder Pattern: Near Mayen, Germany. 2.65 (vs), 7.19 (s), 2.78 (s), 1.93 (s), 2.05 (ms), 3.65 (m), 1.82 (m) Chemistry: (1) (2) (3) TiO2 1.5 1.9 Al2O3 17.2 22.3 13.1 Fe2O3 30.5 27.6 41.9 Cr2O3 0.1 n.d. MgO n.d. n.d. CaO 46.2 44.8 43.7 insol. 4.0 LOI 0.5 Total 94.4 100.3 100.6 (1) Near Mayen, Germany; by semiquantitative spectroscopy. (2) Hatrurim Formation, Israel; corresponds to Ca1.99(Al1.09Fe0.86Ti0.05)Σ=2.00O5. (3) Do.; corresponds to Ca1.95(Fe1.31Al0.64 Ti0.06)Σ=2.01O5. Occurrence: In thermally metamorphosed limestone blocks included in volcanic rocks (near Mayen, Germany); in high-temperature, thermally metamorphosed, impure limestones (Hatrurim Formation, Israel). Association: Calcite, ettringite, wollastonite, larnite, mayenite, gehlenite, diopside, pyrrhotite, grossular, spinel, afwillite, jennite, portlandite, jasmundite (near Mayen, Germany); melilite, mayenite, wollastonite, kalsilite, corundum (Kl¨och, Austria); spurrite, larnite, mayenite (Hatrurim Formation, Israel). -
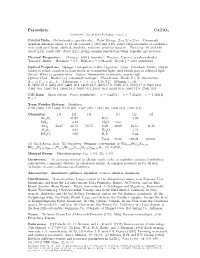
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior
materials Article Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior Ursula Pott 1, Cordula Jakob 2, Daniel Jansen 2, Jürgen Neubauer 2 and Dietmar Stephan 1,* 1 Department of Civil Engineering, Building Materials and Construction Chemistry, Technische Universität Berlin, 13355 Berlin, Germany; [email protected] 2 Department of Geography and Geosciences, GeoZentrum Nordbayern, Friedrich-Alexander-University, 91054 Erlangen, Germany; [email protected] (C.J.); [email protected] (D.J.); [email protected] (J.N.) * Correspondence: [email protected] Received: 2 December 2019; Accepted: 18 February 2020; Published: 21 February 2020 Abstract: The rheological behavior of cement paste and the improvement of its flowability takes center stage in many research projects. An improved flowability can be achieved by the addition of superplasticizers (SP), such as polycarboxylate ethers (PCE). In order to be able to use these PCEs effectively and in a variety of ways and to make them resistant to changes in the environment, it is crucial to understand the influence of SPs on cement hydration. For that reason, the topic of this paper was the incompatibility of a specific SP and an ordinary Portland cement (OPC). The incompatible behavior was analyzed using rheological tests, such as the spread flow test and penetration test, and the behavior was compared by means of an ultrasound technique and explained by the phase content measured by in-situ X-ray diffraction (XRD) the heat evolution measured by calorimetry, and scanning electron microscope (SEM) images. We showed that the addition of the SP in a high dosage led to a prevention of the passivation of the most reactive and aluminum-containing clinker phases, aluminate and brownmillerite.