Aflatoxin Levels in Locally Grown Maize from Makueni District, Kenya J.T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of the East Africa Natural History Society and National Museum
JOURNAL OF THE EAST AFRICA NATURAL HISTORY SOCIETY AND NATIONAL MUSEUM 15 October, 1978 Vol. 31 No. 167 A CHECKLIST OF mE SNAKES OF KENYA Stephen Spawls 35 WQodland Rise, Muswell Hill, London NIO, England ABSTRACT Loveridge (1957) lists 161 species and subspecies of snake from East Mrica. Eighty-nine of these belonging to some 41 genera were recorded from Kenya. The new list contains some 106 forms of 46 genera. - Three full species have been deleted from Loveridge's original checklist. Typhlops b. blanfordii has been synonymised with Typhlops I. lineolatus, Typhlops kaimosae has been synonymised with Typhlops angolensis (Roux-Esteve 1974) and Co/uber citeroii has been synonymised with Meizodon semiornatus (Lanza 1963). Of the 20 forms added to the list, 12 are forms collected for the first time in Kenya but occurring outside its political boundaries and one, Atheris desaixi is a new species, the holotype and paratypes being collected within Kenya. There has also been a large number of changes amongst the 89 original species as a result of revisionary systematic studies. This accounts for the other additions to the list. INTRODUCTION The most recent checklist dealing with the snakes of Kenya is Loveridge (1957). Since that date there has been a significant number of developments in the Kenyan herpetological field. This paper intends to update the nomenclature in the part of the checklist that concerns the snakes of Kenya and to extend the list to include all the species now known to occur within the political boundaries of Kenya. It also provides the range of each species within Kenya with specific locality records . -

The Geomorphology of Southeast Kenya
THE GEOMORPHOLOGY OF SOUTHEAST KENYA A. P. Oosterom STELLINGEN 1. Bij de vorming van de uitgestrekte planatievlakken in Oost-Kenia heeft marine en lacustrine abrasie een belangrijke rol gespeeld. Dit proefschrift. 2. De schaarste aan fossielen van hominiden in Oost-Afrika over de periode van ongeveer 500 000 tot 50 000 jaar BP is schijnbaar. 3. Voor de toepassing van geografische informatiesystemen op bedrijfsniveau in reliefrijke gebieden is het essentieel om programmatuur te ontwikkelen die identificatie van de geologische en geomorfologische positie van een gekozen punt mogelijk maakt. 4. De klink van het Basis- en het Hollandveen is er medeverantwoordelijk voor dat er in Nederland geen aanwijzingen worden gevonden voor hoge holocene zeestanden. 5. De geringschatting van het belang van onderwijs en onderzoek in de geologie en geomorfologie aan de Landbouwuniversiteit te Wageningen is een uiting van onwetenschappelijk doe-het-zelf-denken. 6. Zolang de programmatuur voor het opnemen, opslaan en opvragen van kaarteringsgegevens minder flexibel is dan een veldboekje staat het gebruik van handterminals en veldcomputers vernieuwing in de weg. 7. De betrouwbaarheidsgrens van 25 000 jaar voor de datering van organische carbqnaten met behulp van de 14C-methode is te hoog.. Dit proefschrift. 8. Het woord automatisering wekt onjuiste verwachtingen als het gaat om de invoering en het gebruik van computers. 9. Kerkelijke liedboeken behoren losbladig te zijn. 10. De stelligheid waarmee waarheden worden verdedigd is eerder een maat voor onkunde dan voor inzicht. Stellingen behorende bij het proefschrift: The Geomorphology of Southeast Kenya. A.P. Oosterom Wageningen, 20 april 1988 THE GEOMORPHOLOGY OF SOUTHEAST KENYA Promotoren: Dr. -

Usg Humanitarian Assistance to Kenya
USG HUMANITARIAN ASSISTANCE TO KENYA 35° 36° 37° 38° 39° 40°Original Map Courtesy 41° of the UN Cartographic Section 42° SUDAN The boundaries and names used on this map do not imply official endorsement or acceptance Todenyang COUNTRYWIDE by the U.S. Government. Banya ETHIOPIA Lokichokio KRCS Sabarei a UNICEF 4° F 4° RIFT VALLEY Banissa WFP Ramu Mandera ACTED Kakuma ACRJ Lokwa UNHCR Kangole p ManderaMandera Concern CF Moyale Takaba IFRC North Horr Lodwar IMC F MoyaleMoyale 3° El NORTHWak EASTERN 3° Merlin F Loiyangalani FH FilmAid TurkanaTurkana Buna AC IRC MarsabitMarsabit Mercy USA F J j D Lokichar JRS k WASDA J j Marsabit WajirWajir Tarbaj CARE LWR ikp EASTERNEASTERN Vj J 2° Girito Salesian Missions Lokori Center for 2° S Victims of Torture k Baragoi EASTERN World University Laisamis Wajir of Canada V FH FilmAid WestWest AC PokotPokot NORTHNORTH Handicap Int. RIFTR I F T VALLEYVA L L E Y SamburuSamburu EASTERNEASTERN IRC UGANDA Tot j D Maralal TransTrans NzoiaNzoia MarakwetMarakwet Archer's LWR 1° MtMt Kitale BaringoBaringo Dif ikp 1° Kisima Post Habaswein ElgonElgon NRC LugariLugari Lorule S I WESTERNWESTERN UasinUasin SC BungomaBungoma GishuGishu Mado Gashi G TesoTeso Marigat IsioloIsiolo Busia Webuye Eldoret KeiyoKeiyo Isiolo World UniversityLiboi KakamegaKakamega Lare Kinna of Canada V Burnt Nyahururu LaikipiaLaikipia BusiaBusia Kakamega Forest Butere NandiNandi KoibatekKoibatek (Thomson's Falls) MeruMeru NorthNorth Nanyuki Dadaab SiayaSiaya VihigaVihiga Subukia Mogotio Meru 0° Kipkelion MeruMeru 0° Londiani a KisumuKisumu -

Preliminary Indications of the Effect of Infrastructure Development on Ecosystem Connectivity in Tsavo National Parks, Kenya
FIELD NOTES Preliminary indications of the effect of infrastructure development on ecosystem connectivity in Tsavo National Parks, Kenya Benson Okita-Ouma1,*, Fredrick Lala2,4,*, Richard Moller3, Michael Koskei1, Sospeter Kiambi4, David Dabellen1, Chris Leadismo1, Domnic Mijele4, Jeremiah Poghon2,4, Lucy King1, Frank Pope1, George Wittemyer1,5, Jake Wall1,6, Suzannah Goss7, Robert Obrien2, 4 and Iain Douglas-Hamilton1,8. 1Save The Elephants, PO Box 54667 - 00100, Nairobi, Kenya. 2Tsavo East National Park, PO Box 14 - 80300, Voi Kenya 3Tsavo Trust PO Box 204 - 90128, Mtito Andei, Kenya 4Kenya Wildlife Service, PO Box 40241 - 00100, Nairobi, Kenya. 5Department of Fish, Wildlife, and Conservation Biology Colorado State University 6Lab for Advanced Spatial Analysis, Geography, University of British Columbia, Vancouver, Canada 7National Environmental Management Authority (Lead expert), PO Box 24126, Nairobi, Kenya 8Department of Zoology, Oxford University, United Kingdom *corresponding author: [email protected] and [email protected] Balancing conservation and species. Here we report on a research project that aims infrastructure development to contribute to optimizing the design and manage the existing wildlife crossing structures by monitoring Conserving land and ecosystem connectivity for the movement of elephants in a conservation area of wildlife is increasingly a global challenge as demand global significance that is affected by major–ongoing for infrastructure development to meet growing human and planned–rail and road construction projects. population needs encroaches in many traditional wildlife areas. The survival of wildlife species in arid and semi-arid systems requires interconnected The infrastructure landscapes, and limiting animal movement greatly reduces the system’s ability to sustain viable In 2014 the Kenyan government initiated construction wildlife populations (Vasudev et al. -

Nairobi CBD Pesapoint
PESA POINT ATM LOCATIONS Nairobi CBD PesaPoint PesaPoint at KCB » Eco Fedha Towers, Nairobi » Capital Hill » Guardian Biashara St, » K.E.M.U Meru Nairobi » Kipande House » Nakumatt Lifestyle, Nairobi » Moi Avenue » Uniafric House, Nairobi » Moi referral » Afya Centre » Museum Hill » Banda Street/Koinange » S & L Salama Hse Street » Hilton » Kenyatta Avenue » Moi Avenue, Kenya Cinema Nairobi & Surrounds PesaPoint » Langata Road Kobil, » Nakumatt Ukay, Nairobi » Diamond Plaza, Nairobi Nairobi » Oilibya Lusaka Road, » Eastleigh DTB Branch, » Mall Westlands, Nairobi Nairobi Nairobi » Mater Hospital, Nairobi » Oilibya Saba Saba, » Engen Kahawa Sukari, » Naivas Supermarket, Mombasa Kahawa Kayole, Nairobi » South C Kenol, Nairobi » Kasuku Centre, Nairobi » Total Dagoretti Corner, » Stima Plaza Parklands, » Langata Road Kenol, Nairobi Nairobi Nairobi » Total Mombasa Rd, Nairobi » Uchumi Jipange, Nairobi » Total Mountain View, Nairobi Coast Province PesaPoint » Kilifi PesaPoint at KCB » Likoni Nakumatt, Mombasa » Malindi » Gateway » Likoni Total, Mombasa » Mtwapa » Hola » Mombasa Trade Centre » Bamburi » Kengeleni » Shell Moi Avenue » Changamwe » Kilindini » Kimilili » Kwale » Lamu Nyanza Province PesaPoint PesaPoint at KCB » Rongo » Kisii Nakumatt » Kisii » Homabay » Kisumu EABS Building » Kisii - 2nd ATM » Kehancha » Awendo,Kisumu » Kisumu West » Kisumu » Kisumu Mega City » Migori 3 » Kisumu Mega City » Kisumu Mega Plaza » Ogembo » Oyugis Western Province PesaPoint PesaPoint at KCB » Sondu » Busia » Bondo » Bungoma » Kakamega » Eldoret » Busia -

Opportunities
WILDI N V E S T M E N T OPPORTUNITIES SAFARI LODGES AND ADVENTURE PROSPECTUS INVEST IN KENYA SAFARI LODGES PROSPECTUS INVESTMENT OPPORTUNITIES FOR DEVELOPMENT AND MANAGEMENT OF SAFARI LODGES & FACILITIES IN KENYA’S NATIONAL PARKS 2018 CONTENTS 2 3 PROPOSED TOURISM DEVELOPMENT SITES 34 36 38 40 42 Sibiloi NP Malka Mari NP 4 4 #019 Central Turkana Island NP Mandera Marsabit South Island NP 5#0 Marsabit NR 2 South 2 Turkana NR Wajir West Pokot Losai NR Samburu Mt. Elgon NP Elgeyo #08 Trans Marakwet Nzoia Isiolo Bungoma Uasin Baringo Shaba NR Gishu Busia 15#0 L.Bogoria NR Laikipia 12 Kakamega #0 Nandi Meru #011 ¯ Vihiga 2 Meru NP 0 Siaya #0 0 Nyandarua 18 Kisumu Mt. Kenya NP Ndere Island#0 Tharaka-Nithi Kora NP Aberdare 7 Mt. Kenya NR Kericho Nakuru NP #0 Homa Bay Nyeri Garissa Ruma #0 3 Embu NP #0 6 Kisii Bomet Murang'a Migori Kiambu Arawale Narok Nairobi NP #09 Machakos NR Masai Kitui Mara NR 10 Tana River Boni NR South Tana River Kitui NR Primate NR Dodori NR 2 2 - Lamu - Kajiado Makueni 21 16 #0 Chyulu #01 #0 Hills NP Tsavo Amboseli NP Code Site Name National Park East NP 1 Kithasyu Gate Chyulu Hills NP 14 2 Sirimon Glade Mt. Kenya NP #0 #017 3 Game Farm KWSTI 13 #0 Kilifi 4 4 Malindi Cafeteria Malindi Marine NP #0 Malindi Tsavo Marine NP 5 Sokorta Diko Marsabit NP West NP 6 Nyati Campsite Ruma NP Taita Taveta 7 Tusk Camp Aberdares NP #020 8 Kasawai Gate Mt. -

World Bank Document
Document of The World Bank Public Disclosure Authorized Report No. 26444-KE Public Disclosure Authorized KENYA TRANSPORT SECTOR MEMORANDUM Public Disclosure Authorized VOLUME III Public Disclosure Authorized KENYA TRANSPORT SECTOR MEMORANDUM Volume 3 ANNEXES ii TABLE OF CONTENTS Pages ANNEX 1: Kenya Main Road Network 1. Introduction 1 2. Survey Background 1 3. Road Service Standards: Main Paved Network 2 4. Road Conditions: Main Paved Network 4 5. Traffic Flows: Main Paved Network 5 6. Engineering Assessment: Main Paved Network 5 7. On-Going Engineering Activities 8 8. The Unpaved Road Network 9 9. Road Categories for Maintenance and Intervention Planning 11 10. District Perspectives 11 Annex A: Vehicle Speeds 15 Annex B: Kenya Traffic 17 ANNEX 2: Port of Mombasa: Cargo Clearance 1. Export Clearance 18 2. Import Clearance 19 3. Transit Procedures 23 4. Informal Payments and Streamlining Clearance 23 ANNEX 3" Civil Aviation 1. Kenya Airways: Key Operating Statistics 25 2. Finances 26 3. International Scheduled Services: Foreign Carriers 27 4. Principal Airports: Physical Characteristics 28 5. Minor Airports 29 6. Air Transport: Commercial Aircrafts 30 This Transport Sector Memorandum was prepared on the basis of missions in November, 2001 and mid- 2002, by Mr. Simon Thomas (Senior Transport Economist) in collaboration with Mr. Josphat Sasia (Operations Officer, AFTTR), Mr. David Rudge (Senior Road Engineer, AFTTR), Mr. Yash Pal Kedia (Principal Railways Engineer, AFTTR), Mr. John King (Aviation Consultant) and Mr. Paul Thompson (Port Consultant). The Road Sector Review was undertaken with the active participation and support of the DFID, EU, KfW and SIDA. The views and recommendations contained in the Transport Sector Memorandum are those of the review team and are not necessarily endorsed by the Management of the World Bank ANNEX 1 KENYA MAIN ROAD NETWORK Review of Present Status and Conditions 1. -

Principals' Gender and Management Effectiveness in Secondary
Journal of Education and Practice www.iiste.org ISSN 2222-1735 (Paper) ISSN 2222-288X (Online) Vol.6, No.14, 2015 Principals’ Gender and Management Effectiveness in Secondary Schools: Case of Mtito Andei Division, Kenya Eunice Wangui Matheri South Eastern Kenya University, P.O . Box 170-90200, Kitui, Kenya Email: [email protected] Selpher K. Cheloti(Ph.D) South Eastern Kenya University, P. O. Box 170-90200, Kitui, Kenya Email: [email protected] David M. Mulwa, Ph. D* South Eastern Kenya University, P. O. Box 170-90200, Kitui, Kenya Email: [email protected] Abstract Educational leadership has a critical role in the transformation of society, and for change to happen, effective leaders are key. The purpose of the study was to determine the effects of principals’ gender on management effectiveness in secondary schools in Mtito-Andei Division, Kenya. The study sought to establish the relationship between the Principals’ gender and their effectiveness in management of the discipline, staff, students and school finance. The study used ex-post facto research design. Simple random sampling was used to select the respondents for the study. The sample size was 28 principals and 140 teachers. Data was collected by use of questionnaires and interview schedules and was analyzed by use of descriptive and inferential statistics. Conceptually, the chi-square test of independence statistic was computed. In hypotheses the four scores in management of discipline, management of staff personnel, management of students and management of financial resources were converted from continuous data to discreet data (categories) respectively and then Chi-square used to test the hypotheses. -

A CHECK LIST of PLANTS RECORDED in TSAVO NATIONAL PARK, EAST by P
Page 169 A CHECK LIST OF PLANTS RECORDED IN TSAVO NATIONAL PARK, EAST By P. J. GREENWAY INTRODUCTION A preliminary list of the vascular plants of the Tsavo National Park, Kenya, was prepared by Mr. J. B. Gillett and Dr. D. Wood of the East African Herbarium during 1966. This I found most useful during a two month vegetation survey of Tsavo, East, which I was asked to undertake by the Director of Kenya National Parks, Mr. P. M. Olindo, during "the short rains", December-January 1966-1967. Mr. Gillett's list covered both the East and West Tsavo National Parks which are considered by the Trustees of the Kenya National Parks as quite separate entities, each with its own Warden in Charge, their separate staffs and organisations. As a result of my two months' field work I decided to prepare a Check List of the plants of the Tsavo National Park, East, based on the botanical material collected during the survey and a thorough search through the East African Herbarium for specimens which had been collected previously in Tsavo East or the immediate adjacent areas. This search was started in May, carried out intermittently on account of other work, and was completed in September 1967. BOTANICAL COLLECTORS The first traveller to have collected in the area of what is now the Tsavo National Park, East, was J. M. Hildebrandt who in January 1877 began his journey from Mombasa towards Mount Kenya. He explored Ndara and the Ndei hills in the Taita district, and reached Kitui in the Ukamba district, where he spent three months, returning to Mombasa and Zanzibar in August. -

National Assembly
July 25, 2019 PARLIAMENTARY DEBATES 1 NATIONAL ASSEMBLY OFFICIAL REPORT Thursday, 25th July 2019 The House met at 2.30 p.m. [The Speaker (Hon. Justin Muturi) in the Chair] PRAYERS MESSAGE PASSAGE OF THE DIVISION OF REVENUE BILL Hon. Speaker: Hon. Members, pursuant to the provisions of Standing Order No.41, I wish to report to the House that I have received a Message from the Senate regarding the passage of the Division of Revenue Bill (Senate Bill No.13 of 2019). The Message reads in part: “…the Senate on Tuesday, 23rd July 2019 considered and passed the said Bill without amendments”. The Senate now seeks the concurrence of the National Assembly on the said Bill, in accordance with the provisions of Article 110 (4) of the Constitution. Standing Order No.143(1) requires the Speaker to cause a Bill received from the Senate to be read a First Time upon conveyance of a Message from the Senate referring Bills to the National Assembly. Accordingly, I direct that the Bill be scheduled for First Reading at the next sitting of the House. Article 95(4)(a) of the Constitution provides that the National Assembly determines the allocation of the national revenue between the levels of Government as provided for in Part 4 of Chapter Twelve. In this regard, after First Reading, the Division of Revenue Bill (Senate Bill No.13 of 2019) stands referred to the Budget and Appropriations Committee for the Committee to advise the House on how to proceed with the consideration of the Bill. Next Order. -

THE COUNTY BOUNDARIES BILL, 2021 ARRANGEMENT of CLAUSES Clause PART I - PRELIMINARY 1—Short Title
SPECIAL ISSUE Kenya Gazette Supplement No. 42 (Senate Bills No. 20) REPUBLIC OF KENYA –––––––" KENYA GAZETTE SUPPLEMENT SENATE BILLS, 2021 NAIROBI, 23rd March, 2021 CONTENT Bill for Introduction into the Senate— PAGE The"County"Boundaries"Bill,"2021 ................................................................ 309 PRINTED AND PUBLISHED BY THE GOVERNMENT PRINTER, NAIROBI 309 THE COUNTY BOUNDARIES BILL, 2021 ARRANGEMENT OF CLAUSES Clause PART I - PRELIMINARY 1—Short title. 2—Interpretation. PART II – COUNTY BOUNDARIES 3—County boundaries. 4—Cabinet secretary to keep electronic records. 5—Resolution of disputes through mediation. 6—Alteration of county boundaries. PART III – RESOLUTION OF COUNTY BOUNDARY DISPUTES 7—Establishment of a county boundaries mediation committee. 8—Nomination of members to the committee. 9—Composition of the committee. 10—Removal of a member of the mediation committee. 11—Remuneration and allowances. 12—Secretariat. 13—Role of a mediation committee. 14—Powers of the committee. 15—Report by the Committee. 16—Extension of timelines. 17—Dissolution of a mediation committee. PART IV – ALTERATION OF COUNTY BOUNDARIES 18—Petition for alteration of the boundary of a county. 19—Submission of a petition. ! 1! 310310 The County Boundaries Bill, 2021 20—Consideration of petition by special committee. 21—Report of special committee. 22—Consideration of report of special committee by the Senate. 23—Consideration of report of special committee by the National Assembly. PART V – INDEPENDENT COUNTY BOUNDARIES COMMISSION 24—Establishment of a commission. 25—Membership of the commission. 26—Qualifications. 27—Functions of the commission. 28—Powers of the commission. 29—Conduct of business and affairs of the commission. 30—Independence of the commission. -
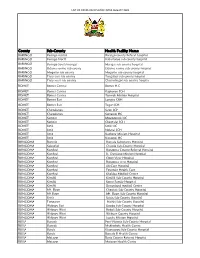
List of Covid-Vaccination Sites August 2021
LIST OF COVID-VACCINATION SITES AUGUST 2021 County Sub-County Health Facility Name BARINGO Baringo central Baringo county Referat hospital BARINGO Baringo North Kabartonjo sub county hospital BARINGO Baringo South/marigat Marigat sub county hospital BARINGO Eldama ravine sub county Eldama ravine sub county hospital BARINGO Mogotio sub county Mogotio sub county hospital BARINGO Tiaty east sub county Tangulbei sub county hospital BARINGO Tiaty west sub county Chemolingot sub county hospital BOMET Bomet Central Bomet H.C BOMET Bomet Central Kapkoros SCH BOMET Bomet Central Tenwek Mission Hospital BOMET Bomet East Longisa CRH BOMET Bomet East Tegat SCH BOMET Chepalungu Sigor SCH BOMET Chepalungu Siongiroi HC BOMET Konoin Mogogosiek HC BOMET Konoin Cheptalal SCH BOMET Sotik Sotik HC BOMET Sotik Ndanai SCH BOMET Sotik Kaplong Mission Hospital BOMET Sotik Kipsonoi HC BUNGOMA Bumula Bumula Subcounty Hospital BUNGOMA Kabuchai Chwele Sub-County Hospital BUNGOMA Kanduyi Bungoma County Referral Hospital BUNGOMA Kanduyi St. Damiano Mission Hospital BUNGOMA Kanduyi Elgon View Hospital BUNGOMA Kanduyi Bungoma west Hospital BUNGOMA Kanduyi LifeCare Hospital BUNGOMA Kanduyi Fountain Health Care BUNGOMA Kanduyi Khalaba Medical Centre BUNGOMA Kimilili Kimilili Sub-County Hospital BUNGOMA Kimilili Korry Family Hospital BUNGOMA Kimilili Dreamland medical Centre BUNGOMA Mt. Elgon Cheptais Sub-County Hospital BUNGOMA Mt.Elgon Mt. Elgon Sub-County Hospital BUNGOMA Sirisia Sirisia Sub-County Hospital BUNGOMA Tongaren Naitiri Sub-County Hospital BUNGOMA Webuye