Ecomorphology, Locomotion, and Microhabitat Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Resurrection of Anolis (Diaphoranolis) Brooksi 1Steven Poe and 2Mason J
Ofcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 11(2) [General Section]: 1–16 (e141). urn:lsid:zoobank.org:pub:31FA8B4B-718B-4440-AE19-9E1AC95524BD Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi 1Steven Poe and 2Mason J. Ryan 1,3Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA 2Arizona Game and Fish Department, 5000 W. Carefree Highway, Phoenix, AZ 85086, USA Abstract.—The spectacular giant anole lizard Anolis insignis is widely distributed but infrequently collected outside of northern Costa Rica. We recently collected several individuals similar to Anolis insignis from localities in Panama and southern Costa Rica. These populations difer from type locality A. insignis in male dewlap color and morphology. We associate one set of these populations with Anolis (Diaphoranolis) brooksi Barbour from Darién, Panama, and describe two additional populations as new species. Keywords. Central America, Costa Rica, lizard, Panama, Reptilia, taxonomy Citation: Poe S and Ryan MJ. 2017. Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi. Amphibian & Reptile Conservation 11(2) [General Section]: 1–16 (e141). Copyright: © 2017 Poe and Ryan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International License, which permits unrestricted use for non-commercial and education purposes only, in any medium, provided the original author and the ofcial and authorized publication sources are recognized and properly credited. The ofcial and authorized publication credit sources, which will be duly enforced, are as follows: ofcial journal title Amphibian & Reptile Conservation; ofcial journal website <amphibian- reptile-conservation.org>. -

Herpetology at the Isthmus Species Checklist
Herpetology at the Isthmus Species Checklist AMPHIBIANS BUFONIDAE true toads Atelopus zeteki Panamanian Golden Frog Incilius coniferus Green Climbing Toad Incilius signifer Panama Dry Forest Toad Rhaebo haematiticus Truando Toad (Litter Toad) Rhinella alata South American Common Toad Rhinella granulosa Granular Toad Rhinella margaritifera South American Common Toad Rhinella marina Cane Toad CENTROLENIDAE glass frogs Cochranella euknemos Fringe-limbed Glass Frog Cochranella granulosa Grainy Cochran Frog Espadarana prosoblepon Emerald Glass Frog Sachatamia albomaculata Yellow-flecked Glass Frog Sachatamia ilex Ghost Glass Frog Teratohyla pulverata Chiriqui Glass Frog Teratohyla spinosa Spiny Cochran Frog Hyalinobatrachium chirripoi Suretka Glass Frog Hyalinobatrachium colymbiphyllum Plantation Glass Frog Hyalinobatrachium fleischmanni Fleischmann’s Glass Frog Hyalinobatrachium valeroi Reticulated Glass Frog Hyalinobatrachium vireovittatum Starrett’s Glass Frog CRAUGASTORIDAE robber frogs Craugastor bransfordii Bransford’s Robber Frog Craugastor crassidigitus Isla Bonita Robber Frog Craugastor fitzingeri Fitzinger’s Robber Frog Craugastor gollmeri Evergreen Robber Frog Craugastor megacephalus Veragua Robber Frog Craugastor noblei Noble’s Robber Frog Craugastor stejnegerianus Stejneger’s Robber Frog Craugastor tabasarae Tabasara Robber Frog Craugastor talamancae Almirante Robber Frog DENDROBATIDAE poison dart frogs Allobates talamancae Striped (Talamanca) Rocket Frog Colostethus panamensis Panama Rocket Frog Colostethus pratti Pratt’s Rocket -

Anfibios Y Reptiles De La Reserva Biológica Alberto Manuel Brenes Amphibians and Reptiles of Alberto Manuel Brenes Biological Reserve
Universidad de Costa Rica - Sede de Occidente Revista Pensamiento Actual - Vol. 15 - No. 25, 2015 ISSN impreso: 1409-0112 / ISSN electrónico: 2215-3586 39 - 59 Ciencias Naturales 39 Anfibios y reptiles de la Reserva Biológica Alberto Manuel Brenes Amphibians and reptiles of Alberto Manuel Brenes Biological Reserve Brayan Heiner Morera Chacón1 / Rónald Sánchez Porras2 Recibido: 25/06/2015 / Aprobado: 20/10/2015 Resumen Se actualizó la lista de anfibios y reptiles de la Reserva Biológica Alberto Manuel Brenes con los cambios taxonómicos más recientes. Además se realizaron 10 giras al campo en los meses de febrero, marzo y abril en las cuales se reportaron 25 nuevas especies para la reserva (seis anfibios y 19 reptiles) para un total de 90 especies reportadas (34 de anfibios y 56 de reptiles). La lista presenta ocho especies de anfibios con una categoría de preocupación mayor según la IUCN de las cuales algunas no se han vuelto a observar en la reserva. Palabras clave: Herpetofauna, anfibios, reptiles, lista, ReBAMB. Abstract The Alberto Manuel Brenes is a small but important nature reserve in Costa Rica. This article presents an updated and illustrated checklist of its amphibians and reptiles based on literature records and 10 expeditions carried out in February, March and April of 2015. There are report 25 new species records (six amphibians, 19 reptiles) for a total of 90 (34 amphibians. 56 reptiles). Eight amphibians belong in the concerned IUCN category and some of them have not been seen in recent years. Key Words: Herpetofauna, amphibians, reptiles, checklist, ReBAMB. Introducción Costa Rica se encuentra entre los países con de su área debido a su gran tamaño (7800 ha) y mayor biodiversidad herpetológica del planeta; se difícil acceso. -

It Is Time for a New Classification of Anoles (Squamata: Dactyloidae)
Zootaxa 3477: 1–108 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Monograph ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:32126D3A-04BC-4AAC-89C5-F407AE28021C ZOOTAXA 3477 It is time for a new classification of anoles (Squamata: Dactyloidae) KIRSTEN E. NICHOLSON1, BRIAN I. CROTHER2, CRAIG GUYER3 & JAY M. SAVAGE4 1Department of Biology, Central Michigan University, Mt. Pleasant, MI 48859, USA. E-mail: [email protected] 2Department of Biology, Southeastern Louisiana University, Hammond, LA 70402, USA. E-mail: [email protected] 3Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA. E-mail: [email protected] 4Department of Biology, San Diego State University, San Diego, CA 92182, USA. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by S. Carranza: 17 May 2012; published: 11 Sept. 2012 KIRSTEN E. NICHOLSON, BRIAN I. CROTHER, CRAIG GUYER & JAY M. SAVAGE It is time for a new classification of anoles (Squamata: Dactyloidae) (Zootaxa 3477) 108 pp.; 30 cm. 11 Sept. 2012 ISBN 978-1-77557-010-3 (paperback) ISBN 978-1-77557-011-0 (Online edition) FIRST PUBLISHED IN 2012 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2012 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. -

Supporting References for Nelson & Ellis
Supplemental Data for Nelson & Ellis (2018) The citations below were used to create Figures 1 & 2 in Nelson, G., & Ellis, S. (2018). The History and Impact of Digitization and Digital Data Mobilization on Biodiversity Research. Publication title by year, author (at least one ADBC funded author or not), and data portal used. This list includes papers that cite the ADBC program, iDigBio, TCNs/PENs, or any of the data portals that received ADBC funds at some point. Publications were coded as "referencing" ADBC if the authors did not use portal data or resources; it includes publications where data was deposited or archived in the portal as well as those that mention ADBC initiatives. Scroll to the bottom of the document for a key regarding authors (e.g., TCNs) and portals. Citation Year Author Portal used Portal or ADBC Program was referenced, but data from the portal not used Acevedo-Charry, O. A., & Coral-Jaramillo, B. (2017). Annotations on the 2017 Other Vertnet; distribution of Doliornis remseni (Cotingidae ) and Buthraupis macaulaylibrary wetmorei (Thraupidae ). Colombian Ornithology, 16, eNB04-1 http://asociacioncolombianadeornitologia.org/wp- content/uploads/2017/11/1412.pdf [Accessed 4 Apr. 2018] Adams, A. J., Pessier, A. P., & Briggs, C. J. (2017). Rapid extirpation of a 2017 Other VertNet North American frog coincides with an increase in fungal pathogen prevalence: Historical analysis and implications for reintroduction. Ecology and Evolution, 7, (23), 10216-10232. Adams, R. P. (2017). Multiple evidences of past evolution are hidden in 2017 Other SEINet nrDNA of Juniperus arizonica and J. coahuilensis populations in the trans-Pecos, Texas region. -

A New Giant Anole (Squamata: Iguanidae: Dactyloinae) from Southwestern Ecuador
Zootaxa 4991 (2): 295–317 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2021 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4991.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:E9FA6C70-379D-490D-A68E-D9354B8D1938 A new giant anole (Squamata: Iguanidae: Dactyloinae) from southwestern Ecuador FERNANDO AYALA-VARELA1,2, SEBASTIÁN VALVERDE1,3, STEVEN POE5, ANDREA E. NARVÁEZ6,7, MARIO H. YÁNEZ-MUÑOZ7,8 & OMAR TORRES-CARVAJAL1,4 * 1Museo de Zoología, Departamento de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador, Avenida 12 de Octubre 1076 y Roca, Apartado 17-01-2184, Quito, Ecuador. 2 �[email protected]; https://orcid.org/0000-0002-1029-2081 3 �[email protected]; https://orcid.org/0000-0001-8125-6726 4 �[email protected]; https://orcid.org/0000-0003-0041-9250 5Department of Biology, University of New Mexico, Albuquerque, NM, 87131, USA. �[email protected]; https://orcid.org/0000-0002-7020-4741 6Facultad de Ciencias Naturales, Universidad de Guayaquil, Av. Raúl Gómez Lince s/n Av. Juan Tanca Marengo, Guayaquil, Ecuador. �[email protected]; https://orcid.org/0000-0002-5953-9233 7Department of Ecology, Environment and Evolution, La Trobe University, Bundoora, Australia. 8Unidad de Investigación, Instituto Nacional de Biodiversidad. Rumipamba 341 y Av. de los Shyris. Apartado 17-07-8976, Quito, Ec- uador. �[email protected]; https://orcid.org/0000-0003-3224-1987 *Corresponding author. �[email protected] Abstract We describe a new species of Anolis lizard from the Pacific slopes of the Andes of southwestern Ecuador at elevations between 372–1,000 m. -

Ecography ECOG-03593 Tarr, S., Meiri, S., Hicks, J
Ecography ECOG-03593 Tarr, S., Meiri, S., Hicks, J. J. and Algar, A. C. 2018. A biogeographic reversal in sexual size dimorphism along a continental temperature gradient. – Ecography doi: 10.1111/ecog.03593 Supplementary material SUPPLEMENTARY MATERIAL A biogeographic reversal in sexual size dimorphism along a continental temperature gradient Appendix 1: Supplementary Tables and Figures Table A1. Placement of species missing from phylogeny. Species Comment Reference Most closely related to oaxaca and Campbell, J.A., et al. 2016. A new species of Abronia mixteca, most similar to mixteca Abronia cuetzpali (Squamata: Anguidae) from the Sierra Madre del Sur of according to Campbell et al. so add Oaxaca, Mexico. Journal of Herpetology 50: 149-156. as sister to mixteca Anolis alocomyos Both formerly part of tropidolepis, Köhler, G., et al. 2014. Two new species of the Norops & Anolis make a random clade with pachypus complex (Squamata, Dactyloidae) from Costa leditzigorum tropidolepis Rica. Mesoamerican Herpetology 1: 254–280. Part of a clade with microtus and Poe S, Ryan M.J. 2017. Description of two new species Anolis brooksi & ginaelisae so make a random clade similar to Anolis insignis (Squamata: Iguanidae) and Anolis kathydayae with these & brooksi & kathydayae, resurrection of Anolis (Diaphoranolis) brooksi. Amphibian based on Poe & Ryan. & Reptile Conservation 11: 1–16. Part of a clade with aquaticus and Köhler, J.J., et al. 2015. Anolis marsupialis Taylor 1956, a Anolis woodi so make a random clade with valid species from southern Pacific Costa Rica (Reptilia, marsupialis these Squamata, Dactyloidae). Zootaxa 3915111–122 Köhler, G., et al. 2016. Taxonomic revision of the Norops Anolis mccraniei, Formerly part of tropidonotus, so tropidonotus complex (Squamata, Dactyloidae), with the Anolis spilorhipis, split tropidonotus into a random resurrection of N. -

Phylogeny of the Dactyloa Clade of Anolis Lizards
Phylogeny of the Dactyloa Clade of Anolis Lizards: New Insights from Combining Morphological and Molecular Data Author(s): María Del Rosario Castañeda and KEVIN DE QUEIROZ Source: Bulletin of the Museum of Comparative Zoology, 160(7):345-398. 2013. Published By: Museum of Comparative Zoology, Harvard University DOI: http://dx.doi.org/10.3099/0027-4100-160.7.345 URL: http://www.bioone.org/doi/full/10.3099/0027-4100-160.7.345 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/ page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non- commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PHYLOGENY OF THE DACTYLOA CLADE OF ANOLIS LIZARDS: NEW INSIGHTS FROM COMBINING MORPHOLOGICAL AND MOLECULAR DATA MARI´A DEL ROSARIO CASTAN˜ EDA1,2,3 AND KEVIN DE QUEIROZ2 CONTENTS Note -

Origins and Biogeography of the Anolis Crassulus Subgroup (Squamata: Dactyloidae) in the Highlands of Nuclear Central America Erich P
Hofmann and Townsend BMC Evolutionary Biology (2017) 17:267 DOI 10.1186/s12862-017-1115-8 RESEARCHARTICLE Open Access Origins and biogeography of the Anolis crassulus subgroup (Squamata: Dactyloidae) in the highlands of Nuclear Central America Erich P. Hofmann1,2* and Josiah H. Townsend1 Abstract Background: Recent studies have begun to reveal the complex evolutionary and biogeographic histories of mainland anoles in Central America, but the origins and relationships of many taxa remain poorly understood. One such group is the Anolis (Norops) crassulus species subgroup, which contains ten morphologically similar highland taxa, the majority of which have restricted distributions. The nominal taxon A. crassulus has a disjunct distribution from Chiapas, Mexico, through Guatemala, in the highlands of El Salvador, and in the Chortís Highlands of Honduras. We test the relationships of these species using multiple mitochondrial and nuclear loci in concatenated and multispecies coalescent frameworks, in an effort to both resolve long-standing taxonomic confusion and present new insights into the evolution and biogeography of these taxa. Results: Sequences of multiple mitochondrial and nuclear loci were generated for eight of the ten species of the Anolis crassulus species subgroup. We analyzed phylogenetic relationships and estimated divergence times and ancestral ranges of the subgroup, recovering a monophyletic subgroup within Anolis. Within the nominal taxon Anolis crassulus, we recovered multiple genetically distinct lineages corresponding to allopatric populations, and show that the Chortís Highland lineage split from the others over 13 MYA. Additionally, distinct mitochondrial lineages are present within the taxa A. heteropholidotus and A. morazani, and importantly, samples of A. crassulus and A. -

A “Precaução Padrão”
ABSTRACTS – 2007 JOINT MEETING OF ICHTHYOLOGISTS & HERPETOLOGISTS COMPLIED BY M.A. DONNELLY (for co-authored abstracts, underlined name = presenter) Abrams, Alyssa Pheromone Production and Volatility in the Copperhead (Agkistrodon contortrix) in Captivity Saint Louis University, St. Louis, MO, United States Snakes were collected during the summer in central Missouri and maintained in captivity at Washington University's Tyson Research Center. Snakes were maintained at 24oC and fed weekly. Beginning in late August females were tested for the presence of pheromones. Male courtship behaviors served as the bioassay for pheromone presence. All experiments were conducted in an outdoor arena lined with a disposable plastic liner. Volatility of pheromones was measured by placing the female in a clean opaque plastic container, containing 3-3mm diameter holes, on a six cm pedestal in the arena containing one male. Male behavior was observed for 30 min after which the female was removed and placed directly in the arena to determine her attractivity. All eight females tested elicited some male courtship behaviors during at least some trials. All males responded to attractive females. Males showed no interest in other males. Male behaviors included: tongue flicking, trailing, head jerking, head bobbing, head poking and tail searching. No mating was observed. In three trials, males were attracted to the cages containing the females, indication the attractivity pheromone may be volatile, however, in tests using skin wipes from attractive females no males responded. An immediately post-partum female was attractive to males. ______________________________________________________________________________ Adams, Cory; Saenz, Daniel; Pierce, Josh Anuran Use of Primary Successional Ponds USDA Forest Service, Southern Research Station, Nacogdoches, TX, United States Freshwater habitats exist along a continuum ranging from short duration ephemeral sites to permanent bodies of water. -

2015 Annual Report Joseph A
University of New Mexico UNM Digital Repository Annual Reports Museum of Southwestern Biology 6-9-2016 2015 Annual Report Joseph A. Cook Follow this and additional works at: https://digitalrepository.unm.edu/msb_annual_reports Recommended Citation Cook, Joseph A.. "2015 Annual Report." (2016). https://digitalrepository.unm.edu/msb_annual_reports/1 This Annual Report is brought to you for free and open access by the Museum of Southwestern Biology at UNM Digital Repository. It has been accepted for inclusion in Annual Reports by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Museum of Southwestern Biology 2015 Annual Report Joseph A. Cook, Director 9 June 2016 1 The University of New Mexico’s Museum of Southwestern Biology The Museum of Southwestern Biology (MSB) provides infrastructure to the Department of Biology at the University of New Mexico and to a world-wide community of scientists, educators, public health workers, and natural resource managers that need information on diverse aspects of biodiversity. Our collections and web- accessible databases, managed by a set of dedicated Collection Managers, constitute an informatics resource that contributes to understanding the complexity of planetary life and related ecosystem function on local, regional, and global scales. High research activity at MSB demonstrates the increasing use of collections (both samples and data) in environmental and biomedical research. Our collections now support a tremendous number of peer- reviewed publications (nearly 200 in 2015) and attract significant grant dollars (> $2.6M at UNM in 2015). The museum is an unparalleled informatics resource contributing to applied efforts in conservation as well as theoretical advancements in biology across time and across local, regional, and global scales. -
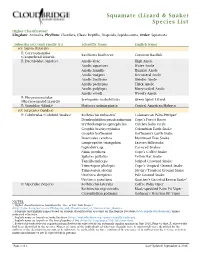
Squamate (Lizard & Snake) Species List
Squamate (Lizard & Snake) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Lepidosauria, Order: Squamata Suborder (sO:) and Family (F:) Scientific Name2 English Name2 sO: Sauria (Lizards) F: Corytophanidae Basiliscus basiliscus Common Basilisk (Casquehead Lizards) F: Dactyloidae (Anoles) Anolis altae High Anole Anolis aquaticus Water Anole Anolis humilis Humble Anole Anolis insignis Decorated Anole Anolis limifrons Slender Anole Anolis pachypus Thick Anole Anolis polylepis Many-scaled Anole Anolis woodi Wood's Anole F: Phrynosomatidae Sceloporus malachiticus Green Spiny Lizard (Phrynosomatid Lizards) F: Scincidae (Skinks) Marisora unimarginata Central American Mabuya sO: Serpentes (Snakes) F: Colubridae (Colubrid Snakes) Bothriechis nubestris5 Talamancan Palm-Pitviper5 Dendrophidion paucicarinatum Cope's Forest Racer Erythrolamprus epinephelus Culebra boba verde Geophis brachycephalus Colombian Earth Snake Geophis hoffmanni Hoffmann's Earth Snake Imantodes cenchoa Blunthead Tree Snake Lampropeltis triangulum Eastern Milksnake Leptodeira sp. Cat-eyed Snakes Ninia psephota Cope's Coffee Snake Spilotes pullatus Yellow Rat Snake Tantilla ruficeps Striped Crowned Snake3 Trimetopon pliolepis Cope's Tropical Ground Snake Trimetopon slevini Slevin's Tropical Ground Snake Urotheca decipiens Pale Ground Snake Urotheca guentheri Gunther's Graceful Brown Snake4 F: Viperidae (Vipers) Bothriechis lateralis Coffee Palm Viper Bothriechis nigroviridis5 Black-speckled Palm Pit Viper5 Cerrophidion godmani Godman's Montane Pit Viper NOTES: 1, Higher classification as found on the Tree of Life Web Project (http://tolweb.org/accessory/Phylogeny_and_Classification_of_Amniotes?acc_id=462). 2, Scientific and English names based on current classifications as found on The Reptile Database (www.reptile-database.org), unless indicated otherwise. 3, English name as found on SnakeDatabase (http://snakedatabase.org/species/tantilla/ruficeps). 4, English name as found on The Encyclopedia of Life (http://eol.org/pages/1055095/overview).