Chemicals for Electronics Industry (March 2001)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet Material Name: TUNGSTEN HEXAFLUORIDE SDS ID: MAT24560
Safety Data Sheet Material Name: TUNGSTEN HEXAFLUORIDE SDS ID: MAT24560 Section 1 - PRODUCT AND COMPANY IDENTIFICATION Material Name TUNGSTEN HEXAFLUORIDE Synonyms MTG MSDS 85; TUNGSTEN FLUORIDE (WF6), (OC-6-11)-; WOLFRAM HEXAFLUORIDE; HEXAFLUOROTUNGSTEN; TUNGSTEN(6+) FLUORIDE; TUNGSTEN HEXAFLUORIDE (WF6); TUNGSTEN VI FLUORIDE; TUNGSTEN FLUORIDE; UN 2196; F6W Chemical Family Fluoride, inorganic, metal Product Use Industrial and Specialty Gas Applications. Restrictions on Use None known. Details of the supplier of the safety data sheet MATHESON TRI-GAS, INC. 3 Mountainview Road Warren, NJ 07059 General Information: 1-800-416-2505 Emergency #: 1-800-424-9300 (CHEMTREC) Outside the US: 703-527-3887 (Call collect) Section 2 - HAZARDS IDENTIFICATION Classification in accordance with paragraph (d) of 29 CFR 1910.1200. Gases Under Pressure - Liquefied gas Acute Toxicity - Inhalation - Gas - Category 2 Skin Corrosion/Irritation - Category 1 Serious Eye Damage/Eye Irritation - Category 1 Specific target organ toxicity - Repeated exposure - Category 1 GHS Label Elements Symbol(s) Signal Word Danger Hazard Statement(s) Contains gas under pressure; may explode if heated. Fatal if inhaled. Causes severe skin burns and eye damage. Causes damage to organs through prolonged or repeated exposure. (bones ) Precautionary Statement(s) Prevention Do not breathe gas. Use only outdoors or in a well-ventilated area. In case of inadequate ventilation wear respiratory protection. ____________________________________________________________ Page 1 of 9 Issue date: 2021-07-07 Revision 7.0 Print date: 2021-07-07 Safety Data Sheet Material Name: TUNGSTEN HEXAFLUORIDE SDS ID: MAT24560 Do not eat, drink or smoke when using this product. Wear protective gloves/protective clothing/eye protection/face protection. -

Tungsten Hexafluoride WF6 Safety Data Sheet SDS P4855
Tungsten hexafluoride Safety Data Sheet P-4855 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/1985 Revision date: 10/24/2016 Supersedes: 04/28/2015 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Name : Tungsten hexafluoride CAS No : 7783-82-6 Formula : WF6 Other means of identification : Tungsten hexafluoride 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Industrial use. Use as directed. 1.3. Details of the supplier of the safety data sheet Praxair, Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA T 1-800-772-9247 (1-800-PRAXAIR) - F 1-716-879-2146 www.praxair.com 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS-US classification Liquefied gas H280 Acute Tox. 2 (Inhalation: gas) H330 Skin Corr. 1A H314 Eye Dam. 1 H318 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS04 GHS05 GHS06 Signal word (GHS-US) : DANGER Hazard statements (GHS-US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED H314 - CAUSES SEVERE SKIN BURNS AND EYE DAMAGE H330 - FATAL IF INHALED CGA-HG11 - SYMPTOMS MAY BE DELAYED CGA-HG22 - CORROSIVE TO THE RESPIRATORY TRACT Precautionary statements (GHS-US) : P202 -

Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev Iowa State University
Chemistry Publications Chemistry 8-2003 Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev Iowa State University Mark S. Gordon Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/chem_pubs Part of the Chemistry Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ chem_pubs/419. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemistry at Iowa State University Digital Repository. It has been accepted for inclusion in Chemistry Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Abstract Titanium dichloride was investigated as a potential catalyst for the bis-silylation reaction of ethylene with hexachlorodisilane. Ab initio electronic structure calculations at the restricted Hartree−Fock (RHF), density functional (DFT), second-order perturbation (MP2), and couple cluster (CCSD) levels of theory were used to find optimized structures, saddle points, and minimum-energy paths that connect them. The er action was found to have a net zero barrier at the DFT, MP2, and CCSD levels of theory. Dynamic correlation is found to be important for this reaction. Disciplines Chemistry Comments Reprinted (adapted) with permission from Organometallics 22 (2003): 4111, doi:10.1021/om0303350. Copyright 2014 American Chemical Society. This article is available at Iowa State University Digital Repository: http://lib.dr.iastate.edu/chem_pubs/419 Organometallics 2003, 22, 4111-4117 4111 Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev and Mark S. -

Gas and Metal Compatibility Guide
Gas & Metal Compatibility Table Chemical Suggested Choosing a Mott High Purity Gas Filter: Gas Formula Filter Media Choosing the best metal filter is not always a simple Ammonia NH 3 SS/Ni matter, because in addition to easily identified variables Argon Ar SS/Ni Arsenic Pentafluoride AsF 5 SS/Ni (i.e., gas, pressure and flow), there are subjective Arsine AsH 3 SS/Ni(1) considerations. Some gases are compatible with more Boron Trichloride BCI 3 Ni/H than one type of metal which allows you a choice when Boron Trifluoride BF 3 Ni/H Carbon Dioxide CO 2 SS/Ni selecting the right filter for your application. Carbon Monoxide * CO SS * Carbon Tetrachloride CCl 4 SS/Ni The information contained in this table is a guideline for Carbon Tetraflouride CF 4 SS/Ni appropriate filter selection. Consultation with your gas Chlorine Cl 2 SS/Ni/H Diborane B2H6 SS/Ni(1) supplier is recommended to ensure Dichlorosilane SiH 2Cl 2 Ni/H gas compatibility. Because so many Diethyltelluride C4H10 Te SS/Ni Fluorine F2 Ni/H factors can affect the chemical Freon 13 CClF 3 SS/Ni resistance of a given product, you Freon 14 Tetrafluoromethane CF 4 SS/Ni should pretest under your own Freon 23 Trifluoromethane/Fluoro-form CHF 3 SS/Ni Freon 115 Chloropentafluoroethane C2ClF 5 SS/Ni operating conditions. Freon 116 Hexafluoroethane C2F6 SS/Ni As with any Germane GeH 4 SS/Ni Helium He SS/Ni chemical Hydrogen H2 SS/Ni application, Hydrogen Bromide HBr Ni/H Hydrogen Chloride HCl Ni/H safety precautions Hydrogen Fluoride HF Ni/H as noted on MSDS Hydrogen Selenide H2Se SS/Ni sheets should be Hydrogen Sulfide H2S SS/Ni Krypton Kr SS/Ni observed . -

Low Pressure Chemical Vapor Deposition of A-Si:H from Disilane Cole Petersburg Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 Low pressure chemical vapor deposition of a-Si:H from disilane Cole Petersburg Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Electrical and Electronics Commons, and the Materials Science and Engineering Commons Recommended Citation Petersburg, Cole, "Low pressure chemical vapor deposition of a-Si:H from disilane" (2007). Retrospective Theses and Dissertations. 14557. https://lib.dr.iastate.edu/rtd/14557 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Low pressure chemical vapor deposition of a-Si:H from disilane by Cole Petersburg A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Electrical Engineering Program of Study Committee: Vikram Dalal, Major Professor Kristen Constant Santosh Pandey Iowa State University Ames, Iowa 2007 Copyright c Cole Petersburg, 2007. All rights reserved. UMI Number: 1443091 UMI Microform 1443091 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii TABLE OF CONTENTS LIST OF TABLES . iv LIST OF FIGURES . -

Comprehensive Electronics Solutions Praxair Offers a Full Suite of Electronics Gases, Equipment and Services
Comprehensive Electronics Solutions Praxair offers a full suite of electronics gases, equipment and services. Applications: Semiconductor From cost effectiveness to supply reliability to product quality – across North LEDs and Photonics America and around the world – Praxair is the gas supply partner with a proven Solar track record of success. Praxair’s experts are uniquely positioned to address the key needs of your operation, helping you remain competitive in today’s Nanotechnology highly complex, global electronics market. Electronics Assembly Product solutions with consistent performance Praxair is your industry-leading electronics solutions provider, backed by the dedicated service and industry know-how of the largest industrial gas company in North America. Whether its cylinder specialty gases for production processes, facility support gases from a microbulk system, or bulk or on-site delivery systems for large quantity demands, electronics manufacturing requires reliable, consistent products and service to get the job done right. Praxair’s complete suite of electronics gases, delivery equipment and services are designed to help you boost productivity and cut costs – crucial to your bottom line. Process gases that support your productivity Praxair’s global track record of supporting electronics producers in semiconductor, LED and electronics assembly markets among others is second to none. Our process gases maximize productivity, reduce process costs and enable new technologies. Count on Praxair for a reliable supply of gases that -

Primer on Spontaneous Heating and Pyrophoricity
NOT MEASUREMENT SENSITIVE DOE‐HDBK‐1081‐2014 Supersedes DOE‐HDBK‐1081‐94 DOE HANDBOOK PRIMER ON SPONTANEOUS HEATING AND PYROPHORICITY U.S. Department of Energy FSC‐6910 Washington, D.C. 20585 DISTRIBUTION STATEMENT: Approved for public release; distribution is unlimited. This document is available on the Department of Energy Technical Standards Program Web page at: http://www.hss.doe.gov/nuclearsafety/ns/techstds/ Key words: Alkali Metals , Aluminum, Arsine, Calcium, Class D Extinguishing Agents, Coal Storage, Combustible Metals, Diborane, Fire, Hafnium, Heating, Hydrazine, Hydrocarbons, Hypergolic, Hypergolic Reaction, Iron, Lithium, Magnesium, Metals, Microbial Heating, NaK, Organic, Oxidizer, Phosphine, Phosphorus, Plutonium, Potassium, Pyrophoric, Pyrophoricity, Pyrophoric Gases, Pyrophoric Reagents, Silane Specific Area, Sodium, Sodium‐Potassium, Specific Surface Area, Spontaneous, Spontaneous Combustion, Steel, Super Oxides, Thorium, Titanium, Uranium, Water Reactive Metals, Zinc, Zirconium FOREWORD The Primer on Spontaneous Heating and Pyrophoricity is approved for use by all DOE Components. It was developed to help Department of Energy (DOE) facility contractors prevent fires caused by spontaneous ignition. Spontaneously ignitable materials include those that ignite because of a slow buildup of heat (spontaneous heating) and those that ignite in air (pyrophoricity). The scientific principles of combustion and how they affect materials known to be spontaneously combustible are explained. The fire hazards of specific spontaneously heating and pyrophoric materials are discussed as well as techniques to prevent their ignition. Suitable fire extinguishing agents are included for most materials as well as safety precautions for storage and handling. The DOE Primers are fundamental handbooks on safety‐related topics of interest in the DOE Complex and are intended as an educational aid for operations and maintenance personnel and others who may have an interest in this topic. -

Low Temperature Chemical Vapor Deposition and Method for Depositing a Tungsten Silicide Film with Improved Uniformity and Reduced Fluorine Concentration
Europaisches Patentamt 19 European Patent Office Office europeen des brevets © Publication number : 0 591 086 A2 12 EUROPEAN PATENT APPLICATION © Application number : 93480132.5 © int. ci.5: H01L 21/3205, C23C 16/44, C23C 16/42, H01L 21/285 @ Date of filing : 10.09.93 The application is published incomplete as filed @ Inventor : Gow, Thomas Richard (Article 93 (2) EPC). The points in the 4 Lyman Drive description at which the omission obviously Williston, VT 05495 (US) occurs have been left blank. Inventor : Lebel, Richard John Box 2255A RD1 © Priority : 30.09.92 US 954783 Sheldon, VT 05483 (US) @ Date of publication of application : @ Representative : Klein, Daniel Jacques Henri 06.04.94 Bulletin 94/14 Compagnie IBM France Departement de Propriete Intellectuelle @ Designated Contracting States : F-06610 La Gaude (FR) DE FR GB © Applicant : International Business Machines Corporation Old Orchard Road Armonk, N.Y. 10504 (US) © Low temperature chemical vapor deposition and method for depositing a tungsten silicide film with improved uniformity and reduced fluorine concentration. © A tungsten silicide (WSix) film is formed by GROWTH RATE ( mg/m i n ) chemical vapor deposition using the reduction of tungsten hexafluoride (WF6) with dichlorosi- O — ro lane (SiH2CI2) referred to as DCS and a reduc- ing agent comprising silane (SiH4), hydrogen, or a mixture thereof. According to the method of the present invention, the WSix film is formed in a CVD reactor operating at a low pressure and at a wafer temperature between about 300 and 550°C. In the preferred embodiment, a thin nucleation layer is deposited before the WSix film. -

HIGH HAZARD GAS Review Date: 09/23/2019
University of Pittsburgh EH&S Guideline Number: 04-021 Safety Manual Subject: Effective Date: 04/19/2017 Page 1 of 9 HIGH HAZARD GAS Review Date: 09/23/2019 STORAGE AND USE OF HIGH HAZARD GAS 1. Definition of High Hazard (HH) Gases For these guidelines, any gas meeting one or more of the following definitions based on International Fire Code (IFC) and National Fire Protection Association (NFPA) standards: 1.1. Flammable gas – a material that is a gas at 68ºF (20ºC) or less at an absolute pressure of 14.7 psi (101.325 kPa) when in a mixture of 13% or less by volume with air, or that has a flammable range at an absolute pressure of 14.7 psi (101.325 kPa) with air of at least 12%, regardless of the lower limit 1.2. Pyrophoric gas – a gas with an autoignition temperature in air at or below 130ºF (54.4ºC) 1.3. Health Hazard 3 (HH3) gas – material that, under emergency conditions and according to the standards, can cause serious or permanent injury 1.4. Health Hazard 4 (HH4) gas – material that, under emergency conditions and according to the standards, can be lethal The storage and usage of a gas or gases meeting any of the above definitions must follow all applicable IFC and NFPA guidelines and the requirements outlined in this document. Consult EH&S for specific guidance on gas mixtures containing corrosive, flammable or poisonous gas components (ex. 1% carbon monoxide/nitrogen, 5% hydrogen sulfide/helium). 2. Notification Requirements Prior to Obtaining High Hazard Gases 2.1. -

AIGA 2005 Meeting
AIGA 2005 Meeting Best Practices in Compressed Gas Emergency Response Eugene Ngai Director of ER & Disposal Technology Air Products Singapore August 30, 2005 ElectronicElectronic SpecialtySpecialty MaterialsMaterials A typical Semiconductor Fab uses over 100 Electronic Specialty Materials (ESM) which include: Electronic Specialty Gases (ESG) Electronic Specialty Liquids (ESL) There are over 300 different Electronic Specialty Materials in common use. These have a wide variety of hazards, with many having multiple hazards. In some cases these are new materials which have limited data. These are supplied in a wide variety of packages ranging from small bubblers (50 gms) to large ISO containers (18,180 kg) Gas ER, Aug 2005 – E. Ngai AirAir ProductsProducts SuppliesSupplies ElectronicElectronic SpecialtySpecialty GasesGases whichwhich havehave aa VarietyVariety ofof HazardsHazards Ammonia Helium Perfluoropropane Argon Hydrogen Phosphine Arsine Hydrogen Bromide Phosphorus Pentafluoride Boron Trichloride Hydrogen Chloride Silane Boron Trifluoride Hydrogen Fluoride Silicon Tetrachloride Carbon Dioxide Hydrogen Iodide Silicon Tetrafluoride Carbon Tetrafluoride Hydrogen Selenide Sulfur Hexafluoride Chlorine Methyl Fluoride Sulfur Tetrafluoride Chlorine Trifluoride Methyltrichlorosilane Tetrafluoromethane Diborane Nitrogen Trichlorosilane Dichlorosilane Nitrogen Trifluoride Trimethylsilane Disilane Nitrous Oxide Tungsten Hexafluoride Fluorine Octafluorocyclobutane Xenon Halocarbon 23, 32, 116 Oxygen Gas ER, Aug 2005 – E. Ngai AirAir ProductsProducts -

Chemical Vapor Deposition of Tungsten Oxide Rein U
APPLIED ORGANOMETALLIC CHEMISTRY, VOL. 12, 155–160 (1998) Chemical Vapor Deposition of Tungsten Oxide Rein U. Kirss* and Lamartine Meda Department of Chemistry, Northeastern University, Boston, MA 02115, USA Crystalline and amorphous thin films of tung- literature contains several reports of electro-opti- sten(VI) oxide can be prepared by chemical cally inactive WO3 films prepared by sputtering, vapor deposition using a variety of volatile evaporation or spray methods,3 although later work precursors below 500 °C. Deposition parameters has yielded electrochromic WO3 films by these 1 for preparation of WO3 films from tungsten methods. No attempt is made here to review the hexacarbonyl [W(CO)6], tungsten hexafluoride literature pertaining to WO3 films prepared by these (WF6), tungsten ethoxides [W(OEt)x, x =5,6] methods. 3 and tetra(allyl)tungsten [W(h -C3H5)4] are sum- The present review focuses on CVD of amor- marized. The electrochromic behavior of these phous and crystalline tungsten oxide films, in- films is comparable with that observed for WO3 cluding thermal CVD, plasma-enhanced CVD films prepared by evaporation, sputtering and (PECVD) and photo-assisted CVD (PACVD). The electrodeposition. # 1998 John Wiley & Sons, principle advantage of chemical vapor deposition Ltd. of electronic materials over other methods is in step Appl. Organometal. Chem. 12, 155–160 (1998) coverage, the absence of radiation damage, throughput and the possibility for selective 4 Keywords: tungsten trioxide; electrochromism; growth. For example, CVD of WO3 occurs at thin films; chemical vapor deposition (CVD) temperatures significantly below those for evapora- ° 5 Received 5 December 1996; accepted 3 March 1997 tion (1300 C). -
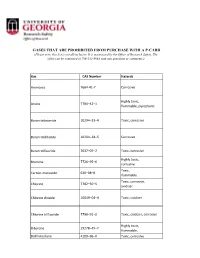
Lists of Gases for Pcard Manual.Xlsx
GASES THAT ARE PROHIBITED FROM PURCHASE WITH A P-CARD (Please note, this list is not all-inclusive. It is maintained by the Office of Research Safety. The office can be contacted at 706-542-9088 with any questions or comments.) Gas CAS Number Hazards Ammonia 7664‐41‐7 Corrosive Highly toxic, Arsine 7784–42–1 flammable, pyrophoric Boron tribromide 10294–33–4 Toxic, corrosive Boron trichloride 10294–34–5 Corrosive Boron trifluoride 7637–07–2 Toxic, corrosive Highly toxic, Bromine 7726–95–6 corrosive Toxic, Carbon monoxide 630–08–0 flammable Toxic, corrosive, Chlorine 7782–50–5 oxidizer Chlorine dioxide 10049–04–4 Toxic, oxidizer Chlorine trifluoride 7790–91–2 Toxic, oxidizer, corrosive Highly toxic, Diborane 19278–45–7 flammable, Dichlorosilane 4109–96–0 Toxic, corrosive Ethylene oxide 75–21–8 Toxic, flammable Highly toxic, corrosive, Fluorine 7782–41–4 oxidizer Highly toxic, Germane 7782–65–2 flammable Hydrogen bromide 10035–10–6 Toxic, corrosive Hydrogen chloride 7647–01–0 Toxic, corrosive Hydrogen cyanide 74–90–8 Highly toxic, flammable Hydrogen fluoride 7664–39–3 Toxic, corrosive Hydrogen iodide 10034‐85‐2 Toxic, corrosive Highly toxic, Hydrogen selenide 7783–07–5 flammable Toxic, flammable, Hydrogen sulfide 7783–06–4 corrosive Methyl bromide 74–83–9 Toxic Methyl isocyanate 624‐83‐9 Highly toxic, flammable Methyl mercaptan 74–93–1 Toxic, flammable Nickel carbonyl 13463–39–3 Highly toxic, flammable Highly toxic, Nitric oxide 10102–43–9 oxidizer Highly toxic, Nitrogen dioxide 10102–44–0 oxidizer, corrosive Highly toxic, Ozone 10028‐15‐6