Conditions of Registration of a Medicine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Germiston South Jurisdiction:- Airport; Albermarle; Asiatic Bazaar; Buhle
Germiston South Jurisdiction:- Airport; Albermarle; Asiatic Bazaar; Buhle Park; Castleview; Cruywagenpark; Dallas Station; Delmenville; Delport; Delville; Denlee; Dewittsrus; Dikatole; Dinwiddie; Driehoek; Egoli Village; Elandshaven; Elsburg; Elspark; Estera; Geldenhuys; Georgetown; Germistoin Industries East; Germiston Industries West; Germiston Lake; Germiston South; Germiston West; Goodhope; Gosforth Park; Graceland; Hazeldene; Hazelpark; Herriotdale; Junction Hill; Jupiter Park Ext 3; Klippoortjie; Klippoortjie Agricultiural Lots; Klippoortjie Park; Knights; Kutalo Hostel; Lake Park; Lambton; Lambton Gardens; Marathon; Mimosa Park; Parkhill Gardens; Pharo Park; Pirowville; Rand Airport; Rondebult; Simmerpan; Summerpark; Tedstoneville; Union Settlements; Wadeville; Webber Germiston North Jurisdiction :- Activia Park; Barvallen; Bedford Gardens; Bedford Park; Bedfordview; Buurendal; Clarens Park; Creston Hill; Dania Park; Dawnview; De Klerkshof; Dowerglen; Dunvegan; Eastliegh; Edenglen; Edenvale; Elandsfontein; Elma Park; Elsieshof; Essexwold; Fisher's Hill; Garden View; Gerdview; Germiston North; Glendower; Greenhills Estate; Harmelia; Henville; Highway Gardens; Homestead; Hurleyvale; Illiondale; Isandovale; Klopperpark; Kruinhof; Malvern East; Maquaksi Plakkers Kamp; Marais-Steyn Park; Marlands; Meadowdale; Morninghill; Oriel; Primrose; Primrose Hill; Primrose Ridge; Rietfontein Hospital; River Ridge; Rustivia; Sebenza; Senderwood; Simmerfield; Solheim; St.Andrews & Exts./Uit; Sunnyridge; Sunnyrock; Symhurst; Symridge; Tunney; Veganview; -

Bedfordview/Edenvale/Greenstone
2019 1 Some detail What is Roots? Reading the charts A landscape survey which spans The community is identified in the 110 metropolitan communities top right corner of the page across South Africa with a total sample of 27 468. Each The sample size, universe size and community is sampled description are displayed at the independently bottom of the page (e.g. (n) 300, representing 40,000 households Formal households are selected or 60,000 shoppers) using multi-stage cluster sampling and purchase decision makers Community data is compared to (shoppers) are randomly the composite of similar selected from the household for communities interview. see below for details In this document The community’s information is always shown in colour and the A community is a defined comparative Metro data in grey geographical footprint from which the samples are drawn. Where applicable community The map provided defines these data is trended back 10 years or boundaries. as far as comparable Large Metros – 62 Communities Medium Metros –18 Communities Small Metros –30 Communities Johannesburg, Cape Town, Tshwane, Nelson Mandela Bay, Bloemfontein, Western Cape (Garden Route, Wine Lands, Ethikwini/Durban, Ekhruleni Pietermaritzburg, West Rand, Vaal, Kimberley, Helderburg) KZN (North and South Coast, Eg: Sandton, Athlone, Durban North, Boksburg, Polokwane, Buffalo City/East London Zululand, Midlands) Mpumalanga (Mbombela, Witbank, Bethal, Middleburg, Ermelo,Lydenburg) Freestate (Welkom, Bethlehem, Kroonstad) Eastern Cape (Uitenhage, Mthatha), Rustenburg 2 Bedfordview -
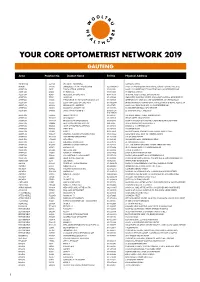
Your Core Optometrist Network 2019 Gauteng
YOUR CORE OPTOMETRIST NETWORK 2019 GAUTENG Area Practice No. Doctor Name Tel No. Physical Address ACTONVILLE 456640 JHETAM N - ACTONVILLE 1539 MAYET DRIVE AKASIA 478490 ENGELBRECHT A J A - WONDERPARK 012 5490086/7 SHOP 404 WONDERPARK SHOPPING C, CNR OF HEINRICH AVE & OL ALBERTON 58017 TORGA OPTICAL ALBERTON 011 8691918 SHOP U 142, ALBERTON CITY SHOPPING MALL, VOORTREKKER ROAD ALBERTON 141453 DU PLESSIS L C 011 8692488 99 MICHELLE AVENUE ALBERTON 145831 MEYERSDAL OPTOMETRISTS 011 8676158 10 HENNIE ALBERTS STREET, BRACKENHURST ALBERTON 177962 JANSEN N 011 9074385 LEMON TREE SHOPPING CENTRE, CNR SWART KOPPIES & HEIDELBERG RD ALBERTON 192406 THEOLOGO R, DU TOIT M & PRINSLOO C M J 011 9076515 ALBERTON CITY, SHOP S03, CNR VOORTREKKER & DU PLESSIS ROAD ALBERTON 195502 ZELDA VAN COLLER OPTOMETRISTS 011 9002044 BRACKEN GARDEN SHOPPING CNTR, CNR DELPHINIUM & HENNIE ALBERTS STR ALBERTON 266639 SIKOSANA J T - ALBERTON 011 9071870 SHOP 23-24 VILLAGE SQUARE, 46 VOORTREKKER ROAD ALBERTON 280828 RAMOVHA & DOWLEY INC 011 9070956 53 VOORTREKKER ROAD, NEW REDRUTH ALBERTON 348066 JANSE VAN RENSBURG C Y 011 8690754/ 25 PADSTOW STREET, RACEVIEW 072 7986170 ALBERTON 650366 MR IZAT SCHOLTZ 011 9001791 172 HENNIE ALBERTS STREET, BRACKENHURST ALBERTON 7008384 GLUCKMAN P 011 9078745 1E FORE STREET, NEW REDRUTH ALBERTON 7009259 BRACKEN CITY OPTOMETRISTS 011 8673920 SHOP 26 BRACKEN CITY, HENNIE ALBERTS ROAD, BRACKENHURST ALBERTON 7010834 NEW VISION OPTOMETRISTS CC 090 79235 19 NEW QUAY ROAD, NEW REDRUTH ALBERTON 7010893 I CARE OPTOMETRISTS ALBERTON 011 9071046 SHOPS -

Xenophobia � Outsider Exclusion Addressing Frail Social Cohesion in South Africa's Diverse Communi�Es
Xenophobia Outsider Exclusion Addressing Frail Social Cohesion in South Africa's Diverse Communies Makause Case Study October 2017 I. INTRODUCTION 1.1 Overview This case study presents findings of a research conducted in May 2016 and May 2017 by the African centre for Migration & Society (ACMS) in Makause, Ekurhuleni Metropolitan Municipality, Gauteng Province. Part of parallel research conducted in 15 other South African communities, this study aims to document and explain the status of social cohesion across the country. More specifically, it aims to identify the causes of group-based conflicts, communal violence, and patterns of violent exclusion. Through these efforts, the research can contribute to developing more effective intervention programmes by relevant stakeholders together with members of the community to promote social cohesion and peaceful cohabitation. The findings presented here are informed by qualitative individual interviews and focus group discussions with residents and relevant key informants conducted between 3 and 7 May 2016 as well as between 2 and 12 May 2017. The research process involved conducting qualitative interviews with relevant stakeholders. A team of four ACMS researchers spent eight days in the area and conducted a total of 35 individual interviews and three focus group discussions. Participants included South Africans and foreign residents as well as key informants including representatives of the local authority, community leaders, faith-based, and other community based organisations. We rely on secondary literature to provide additional background and insight. Recognising that perceptions drive social interactions and mobilisation as much as facts1, our approach documents, and where possible, distinguishes between the two. 1.2 Primary Findings and Conclusions Makause is a socially, economically and politically complex space due primarily to the fact that all its residents are considered illegal dwellers living on a disused and privately owned mine shaft which was never demarcated as a human resettlement area. -

COVID-19 Laboratory Testing Sites
Care | Dignity | Participation | Truth | Compassion COVID-19 Laboratory testing sites Risk assessment, screening and laboratory testing for COVID-19 The information below should give invididuals a clear understanding of the process for risk assessment, screening and laboratory testing of patients, visitors, staff, doctors and other healthcare providers at Netcare facilities: • Risk assessment, screening and laboratory testing of ill individuals Persons who are ill are allowed access to the Netcare facility via the emergency department for risk assessment and screening. Thereafter the person will be clinically assessed by a doctor and laboratory testing for COVID-19 will subsequently be done if indicated. • Laboratory testing of persons sent by external doctors for COVID-19 laboratory testing at a Netcare Group facility Individuals who have been sent to a Netcare Group facility for COVID-19 laboratory testing by a doctor who is not practising at the Netcare Group facility will not be allowed access to the laboratories inside the Netcare facility, unless the person requires medical assistance. This brochure which contains a list of Ampath, Lancet and Pathcare laboratories will be made available to individuals not needing medical assistance, to guide them as to where they can have the testing done. In the case of the person needing medical assistance, they will be directed to the emergency department. No person with COVID-19 risk will be allowed into a Netcare facility for laboratory testing without having consulted a doctor first. • Risk assessment and screening of all persons wanting to enter a Netcare Group facility Visitors, staff, external service providers, doctors and other healthcare providers are being risk assessed at established points outside of Netcare Group hospitals, primary care centres and mental health facilities, prior to them entering the facility. -

Profile: City of Ekurhuleni
2 PROFILE: CITY OF EKURHULENI PROFILE: CITY OF EKURHULENI 3 CONTENT 1. Executive Summary ........................................................................................... 5 2. Introduction: Brief Overview............................................................................. 6 2.1 Historical Perspective ............................................................................................... 6 2.1 Location ................................................................................................................... 7 2.2. Spatial Integration ................................................................................................. 8 3. Social Development Profile............................................................................... 9 3.1 Key Social Demographics ........................................................................................ 9 3.2 Health Profile .......................................................................................................... 12 3.3 COVID-19 .............................................................................................................. 13 3.4 Poverty Dimensions ............................................................................................... 15 3.4.1 Distribution .......................................................................................................... 15 3.4.2 Inequality ............................................................................................................. 16 3.4.3 Employment/Unemployment -

Gauteng Germiston South Sheriff Service Area of Ekurhuleni Central Magisterial District Germiston South Sheriff Service Area Of
!C ! ^ ! !C ^ ! ! ! ! ! ! ! ! ! !C ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! !C ! ! ! ! ! ^ ! !C ! ! $ ^ ! ! !C ! ^ ! !C !C ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! !C !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C !ñ ! ! ! ! ! ! ! ! ^ ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ^ ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ^ ! !C ! $ ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! !C ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! !C ! !C $ ! ! ^ ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! !C ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !. ! ^ ! !C ñ ! ! ! ! ! ! ^ ! ^ ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! !C ! ! !C ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !C ! ! ! ! ! ! ! ! ^ ! ! ^ ! ! ^ ! ! ! ^ ! ! ! ! ! !C ! ! ! ! ! ! ! ! !C ! !C ! ! ^ ! !C ! ! ! ! ñ !C ! !. ! ! ! ! ! ! !C ! ! ! ! ! ! !C ñ !. ! ^ ! ! ! ! ! !C ! !C ! $!C ! ! !. ^ !. ^ ! !C ^ ! ! !C !C ! ! ! !C ! ! !C ! ! ! ! ! ! ! !C ! !C ! !. ^ ! $ ^ !C ! ! !C ^ ! ñ!C !. ! ! !C ^ ! ! !. $ !C !C ! ! ! ! ! ! !C ! !C !. ! ñ ! ! ^ ! !C $ ^ ! ^ ! $ ! ! ! ! !C ! ! ! !C ! ! ! ! ! !C ! ! ! ! ! ! ! ! ! ^ ! !C ! !. ñ ! ! ! ^ !C ! ! !C ! ! ! !C !C ! !C ! ! !C !C ! ! !C ! ! ! ! !C ! ! ! ! !C !C ! ^ ! ! $ !C ! !C ! !C !. ^ ! $ ! !C ! ! ! !C !C ! ! -

35409 8-6 Health
Government Gazette Staatskoerant REPUBLIC OF SOUTH AFRICA REPUBLIEK VAN SUID-AFRIKA June Vol. 564 Pretoria, 2012 8 Junie No. 35409 N.B. The Government Printing Works will not be held responsible for the quality of “Hard Copies” or “Electronic Files” submitted for publication purposes AIDS HELPLINE: 0800-0123-22 Prevention is the cure G12-072103—A 35409—1 2 No. 35409 GOVERNMENT GAZETTE, 8 JUNE 2012 IMPORTANT NOTICE The Government Printing Works will not be held responsible for faxed documents not received due to errors on the fax machine or faxes received which are unclear or incomplete. Please be advised that an “OK” slip, received from a fax machine, will not be accepted as proof that documents were received by the GPW for printing. If documents are faxed to the GPW it will be the sender’s respon- sibility to phone and confirm that the documents were received in good order. Furthermore the Government Printing Works will also not be held responsible for cancellations and amendments which have not been done on original documents received from clients. CONTENTS INHOUD Page Gazette Bladsy Koerant No. No. No. No. No. No. GENERAL NOTICE ALGEMENE KENNISGEWING Health, Department of Gesondheid, Departement van Algemene Kennisgewing General Notice 455 Wet op Beheer van Medisyne en 455 Medicines and Related Substances Act Verwante Stowwe (101/1965): (101/1965): Medicines Control Council: Medisynebeheerraad: Voorwaardes vir Conditions of registration of a medicine die registrasie van 'n medisyne in terme in terms of the provisions of section 15(7) van die bepalings van artikel 15 (7) van of the Act ................................................ -

Province Physical Town
PROVINCE PHYSICAL TOWN PHYSICAL SUBURB PHYSICAL ADDRESS PRACTICE NAME CONTACT NUMBER SPECIALITY PRACTICE NUMBER GAUTENG ALBERTON ALBERTON 34 GENERAL ALBERTS AVENUE BEZUIDENHOUT DENTISTRY Inc 011 827 6913 GENERAL DENTAL PRACTICE 55042 GAUTENG ALBERTON ALBERTON 48 CLINTON ROAD BARTKUNSKY M H 011 907 8810 GENERAL DENTAL PRACTICE 294764 GAUTENG ALBERTON ALBERTON 48 CLINTON ROAD DOS SANTOS PEDRO M M 011 907 8810 GENERAL DENTAL PRACTICE 294764 GAUTENG ALBERTON ALBERTON 48 CLINTON ROAD DU TOIT J P 011 907 8810 GENERAL DENTAL PRACTICE 294764 GAUTENG ALBERTON ALBERTON 7 PADSTOW ROAD HÖLL A J 011 907 8344 GENERAL DENTAL PRACTICE 303526 GAUTENG ALBERTON ALBERTON 7 PADSTOW STREET Dr NIMISHA OOKA 011 907 8344 GENERAL DENTAL PRACTICE 612553 GAUTENG ALBERTON ALBERTON 103 HENNIE ALBERTS STREET PEARSON M S M 011 867 1101 GENERAL DENTAL PRACTICE 5420970 GAUTENG ALBERTON ALBERTON HENNIE ALBERTS ST VELLEMAN R A 011 867 3520 GENERAL DENTAL PRACTICE 5426693 GAUTENG ALBERTON ALBERTON 49 BODMIN STREET ROOS D A 011 869 7133 GENERAL DENTAL PRACTICE 5429153 GAUTENG ALBERTON ALBERTON 7 PADSTOW STREET VOLSCHENK A 011 907 8355 GENERAL DENTAL PRACTICE 5431778 GAUTENG ALBERTON BRACKENHURST 29 VERMOOTEN STREET LEWIS A J 011 867 5970 GENERAL DENTAL PRACTICE 5422035 GAUTENG ALBERTON NEW REDRUTH 49 BODMIN STREET TALJAARD C 011 869 7133 GENERAL DENTAL PRACTICE 211796 GAUTENG ALBERTON RANDHART 64 MICHELLE AVENUE Dr PENNI VAN VUUREN 011 869 0042 GENERAL DENTAL PRACTICE 346926 GAUTENG ALBERTON SOUTH CREST 46 VOORTREKKER ROAD DAWOOD K A S 011 869 6406 GENERAL DENTAL PRACTICE -

For More Information, Contact the Office of the Hod: • 011 999 3845/6194 Introduction
The City of Ekurhuleni covers an extensive area in the eastern region of Gauteng. This extensive area is home to approximately 3.1 million and is a busy hub that features the OR Tambo International Airport, supported by thriving business and industrial activities. Towns that make up the City of Ekurhuleni are Greater Alberton, Benoni, Germiston, Duduza, Daveyton, Nigel, Springs, KwaThema, Katlehong, Etwatwa, Kempton Park, Edenvale, Brakpan, Vosloorun, Tembisa, Tsakane, and Boksburg. Ekurhuleni region accounts for a quarter of Gauteng’s economy and includes sectors such as manufacturing, mining, light and heavy industry and a range of others businesses. Covering such a large and disparate area, transport is of paramount importance within Ekurhuleni, in order to connect residents to the business areas as well as the rest of Gauteng and the country as a whole. Ekurhuleni is highly regarded as one of the main transport hubs in South Africa as it is home to OR Tambo International Airport; South Africa’s largest railway hub and the Municipality is supported by an extensive network of freeways and highways. In it features parts of the Maputo Corridor Development and direct rail, road and air links which connect Ekurhuleni to Durban; Cape Town and the rest of South Africa. There are also linkages to the City Deep Container terminal; the Gautrain and the OR Tambo International Airport Industrial Development Zone (IDZ). For more information, contact the Office of the HoD: • 011 999 3845/6194 Introduction The City of Ekurhuleni covers an extensive area in the eastern region of Gauteng. This extensive area is home to approximately 3.1 million and is a busy hub that features the OR Tambo International Airport, supported by thriving business and industrial activities. -

Spatial Planning Directorate December 2012
CITY PLANNING DEPARTMENT – SPATIAL PLANNING DIRECTORATE DECEMBER 2012 1 REGIONAL SPATIAL DEVELOPMENT FRAMEWORK REGION A EKURHULENI METROPOLITAN MUNICIPALITY SPATIAL CONCEPT December 2012 Commissioned by Drafted by Ekurhuleni Metropolitan Municipality Metroplan Town and Regional Planners 2 TABLE OF CONTENT 4.2 Open Space Network ............................................................... 14 5 NODAL STRUCTURE .......................................................................... 17 5.1 MSDF Proposals .......................................................................... 17 1 INTRODUCTION .................................................................................... 1 5.1.1 Primary Nodes ................................................................... 17 1.1 Aim and Objectives ...................................................................... 1 5.1.2 Secondary Nodes .............................................................. 18 1.2 The Study Area ............................................................................. 2 5.1.3 Station Nodes ..................................................................... 21 1.3 Structure of the Document ........................................................... 2 5.1.4 Combined MSDF Nodes ................................................... 22 2 PROJECT BACKGROUND ................................................................... 4 5.2 Proposed Nodes ......................................................................... 22 3 MAIN FINDINGS OF THE STATUS QUO ANALYSIS -

City Suburb Postalcode Zone ALBERTON MEYERSDAL 1447
City Suburb PostalCode Zone ALBERTON MEYERSDAL 1447 Local ALBERTON ALBERTSDAL 1448 Local ALBERTON ALBERTSDAL EXT 17 1448 Local ALBERTON ALBERTSDAL EXT 18 1448 Local ALBERTON ALBERTSDAL EXT 6 1448 Local ALBERTON BRACKEN DOWNS 1448 Local ALBERTON BRACKEN DOWNS EXT 1 1448 Local ALBERTON BRACKEN DOWNS EXT 2 1448 Local ALBERTON BRACKEN DOWNS EXT 3 1448 Local ALBERTON BRACKEN DOWNS EXT 4 1448 Local ALBERTON BRACKEN DOWNS EXT 5 1448 Local ALBERTON BRACKENHURST 1448 Local ALBERTON BRACKENHURST EXT 1 1448 Local ALBERTON BRACKENHURST EXT 2 1448 Local ALBERTON MABALIA 1448 Local ALBERTON MAYBERRY PARK 1448 Local ALBERTON MAYBERRY PARK EXT 1 1448 Local ALBERTON MEYERSDAL 1448 Local ALBERTON ALBERANTE 1449 Local ALBERTON ALBERANTE EXT 1 1449 Local ALBERTON ALBERTON 1449 Local ALBERTON ALBERTON EXT 28 1449 Local ALBERTON ALBERTON NORTH 1449 Local ALBERTON ALBERTON-NOORD 1449 Local ALBERTON ELANDSHAVEN 1449 Local ALBERTON FLORENTIA 1449 Local ALBERTON GENERAAL ALBERTSPARK 1449 Local ALBERTON NEW REDRUTH 1449 Local ALBERTON NEWMARKET 1449 Local ALBERTON NEWMARKET PARK 1449 Local ALBERTON NEWMARKET PARK EXT 1 1449 Local ALBERTON RACEVIEW 1449 Local ALBERTON RANDHART 1449 Local ALBERTON RANDHART EXT 1449 Local ALBERTON RANDHART EXT 1 1449 Local ALBERTON RANDHART EXT 2 1449 Local ALBERTON RANDHART EXT 4 1449 Local ALBERTON SOUTH CREST 1449 Local ALBERTON VERWOERDPARK 1449 Local ALBERTON ALRODE 1451 Local ALBERTON ALRODE SOUTH EXT 1451 Local ALBERTON BRACKEN GARDENS 1452 Local ALBERTON VERWOERDPARK 1453 Local ALBERTON EDEN PARK 1455 Local ALBERTON ALBERTON