Understanding Eye Movements and Attentional Mechanisms Is Key to Improving Amblyopia Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception
The Journal of Neuroscience, January 28, 2015 • 35(4):1493–1504 • 1493 Behavioral/Cognitive Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception Elena Pretegiani,1,2 Corina Astefanoaei,3 XPierre M. Daye,1,4 Edmond J. FitzGibbon,1 Dorina-Emilia Creanga,3 Alessandra Rufa,2 and XLance M. Optican1 1Laboratory of Sensorimotor Research, NEI, NIH, DHHS, Bethesda, Maryland, 20892-4435, 2EVA-Laboratory, University of Siena, 53100 Siena, Italy, 3Alexandru Ioan Cuza University, Physics Faculty, 700506 Iasi, Romania, and 4Institut du cerveau et de la moelle´pinie e `re (ICM), INSERM UMRS 975, 75013 Paris, France We move our eyes to explore the world, but visual areas determining where to look next (action) are different from those determining what we are seeing (perception). Whether, or how, action and perception are temporally coordinated is not known. The preparation time course of an action (e.g., a saccade) has been widely studied with the gap/overlap paradigm with temporal asynchronies (TA) between peripheral target onset and fixation point offset (gap, synchronous, or overlap). However, whether the subjects perceive the gap or overlap, and when they perceive it, has not been studied. We adapted the gap/overlap paradigm to study the temporal coupling of action and perception. Human subjects made saccades to targets with different TAs with respect to fixation point offset and reported whether they perceived the stimuli as separated by a gap or overlapped in time. Both saccadic and perceptual report reaction times changed in the same way as a function of TA. The TA dependencies of the time change for action and perception were very similar, suggesting a common neural substrate. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -

Acquired Monocular Vision Rehabilitation Program
Volume 44, Number 4, 2007 JRRDJRRD Pages 593–598 Journal of Rehabilitation Research & Development Acquired Monocular Vision Rehabilitation program Carolyn Ihrig, OD;1–2* Daniel P. Schaefer, MD, FACS3–4 1Buffalo Department of Veterans Affairs Medical Center, Buffalo, NY; 2Department of Ophthalmology, Division of Low Vision, 3Department of Otolaryngology, and 4Department of Ophthalmology, Division of Oculoplastic, Orbital Plastics and Reconstructive Surgery, Ira G. Ross Eye Institute, University at Buffalo School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY Abstract—Existing programs concerning patients with low to help with the adjustment to the loss of depth percep- vision do not readily meet the needs of the patient with tion, training on safety and social concerns, and support- acquired monocular vision. This article illustrates the develop- ive counseling would have been beneficial [1]. ment, need, and benefits of an Acquired Monocular Vision Currently, low-vision rehabilitation programs are Rehabilitation evaluation and training program. This proposed available for the partially sighted, such as those suffering program will facilitate the organization of vision rehabilitation from macular degeneration, but they do not readily meet with eye care professionals and social caseworkers to help the needs of the patient with acquired monocular vision. patients cope with, as well as accept, and recognize obstacles they will face in transitioning suddenly to monocular vision. The survey just noted inspired the development of the Acquired Monocular Vision Rehabilitation (AMVR) evaluation and training program to guide and teach these specific skills to each patient with acquired monocular Key words: acquired monocular vision, acquired monocular vision. -
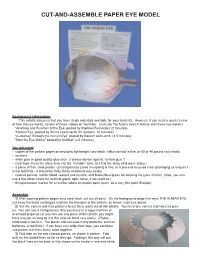
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

The Evolution of Human Intelligence and the Coefficient of Additive Genetic Variance in Human Brain Size ⁎ Geoffrey F
Intelligence 35 (2007) 97–114 The evolution of human intelligence and the coefficient of additive genetic variance in human brain size ⁎ Geoffrey F. Miller a, , Lars Penke b a University of New Mexico, USA b Institut für Psychologie, Humboldt-Universität zu Berlin, Germany Received 3 November 2005; received in revised form 17 August 2006; accepted 18 August 2006 Available online 12 October 2006 Abstract Most theories of human mental evolution assume that selection favored higher intelligence and larger brains, which should have reduced genetic variance in both. However, adult human intelligence remains highly heritable, and is genetically correlated with brain size. This conflict might be resolved by estimating the coefficient of additive genetic variance (CVA) in human brain size, since CVAs are widely used in evolutionary genetics as indexes of recent selection. Here we calculate for the first time that this CVA is about 7.8, based on data from 19 recent MRI studies of adult human brain size in vivo: 11 studies on brain size means and standard deviations, and 8 studies on brain size heritabilities. This CVA appears lower than that for any other human organ volume or life-history trait, suggesting that the brain has been under strong stabilizing (average-is-better) selection. This result is hard to reconcile with most current theories of human mental evolution, which emphasize directional (more-is-better) selection for higher intelligence and larger brains. Either these theories are all wrong, or CVAs are not as evolutionarily informative as most evolutionary geneticists believe, or, as we suggest, brain size is not a very good index for understanding the evolutionary genetics of human intelligence. -

Arteriovenous Dissection in a Living Human
Vienna, Austria, 1990. Dordrecht, Holland: Klu- Table 2. Ultrasonographic Findings of 25 Well-Documented Patients wer Academic Publishers; 1993:307-311. With Cavitary Melanoma of the Uvea in English Literature 9. Frazier-Byrne S, Green RL. Intraocular tumors. In: Frazier-Byrne S, Green RL, eds. Ultrasound of the Eye and Orbit. 2nd ed. St Louis, Mo: Ultrasonographic Findings Mosby; 2002:115-190. 10. Scott CT, Holland GN, Glasgow BJ. Cavita- Solid % Mass tion in ciliary body melanoma. Am J Ophthalmol. Component Loculation Echoes in Septa in Thickness Occupied 1997;123:269-271. Source Present on USG Cavitation Cavitation by Cavity 11. Cohen PR, Rapini RP. Nevus with cyst: a re- port of 93 cases. Am J Dermatopathol. 1993; Kennedy5 NA NA NA NA NA 15:229-234. NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA Reese6 NA NA NA NA NA Arteriovenous Dissection Zakka et al7 ϩ Unilocular ϩ −NA in a Living Human Eye: Stone and Shapiro4 ϩ Unilocular ϩ −65 ϩ Unilocular ϩ −75 Clinicopathologic − Unilocular ϩ −60 Correlation ϩ Unilocular − − 30 ϩ Multilocular ϩϩ 40 Fledelius et al8 − Unilocular − − 75 Although the visual results after ar- Scott et al10 − Multilocular ϩϩ NA teriovenous dissection (AVD) seem 1,2 Lois et al2 − Unilocular ϩ −79 encouraging, its effectiveness has ϩ Unilocular − − 59 not been proved in a controlled, pro- ϩ Multilocular − ϩ 31 spective clinical trial. The role of sur- ϩ Multilocular ϩϩ 59 gical decompression itself remains ϩ − Unilocular −64 unclear,3 and little is known about − Multilocular ϩϩ 62 ϩ Unilocular ϩ −55 surgically induced nerve fiber de- ϩ Multilocular ϩϩ 38 fects. -

Relative Importance of Binocular Disparity and Motion Parallax for Depth Estimation: a Computer Vision Approach
remote sensing Article Relative Importance of Binocular Disparity and Motion Parallax for Depth Estimation: A Computer Vision Approach Mostafa Mansour 1,2 , Pavel Davidson 1,* , Oleg Stepanov 2 and Robert Piché 1 1 Faculty of Information Technology and Communication Sciences, Tampere University, 33720 Tampere, Finland 2 Department of Information and Navigation Systems, ITMO University, 197101 St. Petersburg, Russia * Correspondence: pavel.davidson@tuni.fi Received: 4 July 2019; Accepted: 20 August 2019; Published: 23 August 2019 Abstract: Binocular disparity and motion parallax are the most important cues for depth estimation in human and computer vision. Here, we present an experimental study to evaluate the accuracy of these two cues in depth estimation to stationary objects in a static environment. Depth estimation via binocular disparity is most commonly implemented using stereo vision, which uses images from two or more cameras to triangulate and estimate distances. We use a commercial stereo camera mounted on a wheeled robot to create a depth map of the environment. The sequence of images obtained by one of these two cameras as well as the camera motion parameters serve as the input to our motion parallax-based depth estimation algorithm. The measured camera motion parameters include translational and angular velocities. Reference distance to the tracked features is provided by a LiDAR. Overall, our results show that at short distances stereo vision is more accurate, but at large distances the combination of parallax and camera motion provide better depth estimation. Therefore, by combining the two cues, one obtains depth estimation with greater range than is possible using either cue individually. -

Root Eye Dictionary a "Layman's Explanation" of the Eye and Common Eye Problems
Welcome! This is the free PDF version of this book. Feel free to share and e-mail it to your friends. If you find this book useful, please support this project by buying the printed version at Amazon.com. Here is the link: http://www.rooteyedictionary.com/printversion Timothy Root, M.D. Root Eye Dictionary A "Layman's Explanation" of the eye and common eye problems Written and Illustrated by Timothy Root, M.D. www.RootEyeDictionary.com 1 Contents: Introduction The Dictionary, A-Z Extra Stuff - Abbreviations - Other Books by Dr. Root 2 Intro 3 INTRODUCTION Greetings and welcome to the Root Eye Dictionary. Inside these pages you will find an alphabetical listing of common eye diseases and visual problems I treat on a day-to-day basis. Ophthalmology is a field riddled with confusing concepts and nomenclature, so I figured a layman's dictionary might help you "decode" the medical jargon. Hopefully, this explanatory approach helps remove some of the mystery behind eye disease. With this book, you should be able to: 1. Look up any eye "diagnosis" you or your family has been given 2. Know why you are getting eye "tests" 3. Look up the ingredients of your eye drops. As you read any particular topic, you will see that some words are underlined. An underlined word means that I've written another entry for that particular topic. You can flip to that section if you'd like further explanation, though I've attempted to make each entry understandable on its own merit. I'm hoping this approach allows you to learn more about the eye without getting bogged down with minutia .. -

Geometry and Control of Human Eye Movements Ashoka D
170 IEEE TRANSACTIONS ON AUTOMATIC CONTROL, VOL. 52, NO. 2, FEBRUARY 2007 Geometry and Control of Human Eye Movements Ashoka D. Polpitiya, Member, IEEE, Wijesuriya P. Dayawansa, Fellow, IEEE, Clyde F. Martin, Fellow, IEEE, and Bijoy K. Ghosh, Fellow, IEEE Abstract—In this paper, we study the human oculomotor system as a simple mechanical control system. It is a well known physio- logical fact that all eye movements obey Listing’s law, which states that eye orientations form a subset consisting of rotation matrices for which the axes are orthogonal to the normal gaze direction. First, we discuss the geometry of this restricted configuration space (re- ferred to as the Listing space). Then we formulate the system as a simple mechanical control system with a holonomic constraint. We propose a realistic model with musculotendon complexes and ad- dress the question of controlling the gaze. As an example, an optimal energy control problem is formulated and numerically solved. Index Terms—Eye movements, geodesics, Hill model, Listing’s law, simple mechanical control systems. I. INTRODUCTION IOLOGICAL systems are becoming more appealing to ap- Bproaches that are commonly used in systems theory and suggest new design principles that may have important prac- tical applications in manmade systems. The principles of con- Fig. 1. Anatomy of the eye (courtesy of Yale University School of Medicine). trol theory are central to many of the key questions in biological engineering. Eye movements, for an example, reflect how the brain and the musculotendon system work in unison to control ignored but focusing on the information processing and control the gaze directions while ensuring that attitudes are confined to aspects [5], [6]. -

A Pictorial Anatomy of the Human Eye/Anophthalmic Socket: a Review for Ocularists
A Pictorial Anatomy of the Human Eye/Anophthalmic Socket: A Review for Ocularists ABSTRACT: Knowledge of human eye anatomy is obviously impor- tant to ocularists. This paper describes, with pictorial emphasis, the anatomy of the eye that ocularists generally encounter: the anophthalmic eye/socket. The author continues the discussion from a previous article: Anatomy of the Anterior Eye for Ocularists, published in 2004 in the Journal of Ophthalmic Prosthetics.1 Michael O. Hughes INTRODUCTION AND RATIONALE B.C.O. Artificial Eye Clinic of Washington, D.C. Understanding the basic anatomy of the human eye is a requirement for all Vienna, Virginia health care providers, but it is even more significant to eye care practition- ers, including ocularists. The type of eye anatomy that ocularists know, how- ever, is more abstract, as the anatomy has been altered from its natural form. Although the companion eye in monocular patients is usually within the normal range of aesthetics and function, the affected side may be distorted. While ocularists rarely work on actual eyeballs (except to cover microph- thalmic and blind, phthisical eyes using scleral cover shells), this knowledge can assist the ocularist in obtaining a naturally appearing prosthesis, and it will be of greater benefit to the patient. An easier exchange among ocularists, surgeons, and patients will result from this knowledge.1, 2, 3 RELATIONSHIPS IN THE NORMAL EYE AND ORBIT The opening between the eyelids is called the palpebral fissure. In the nor- mal eye, characteristic relationships should be recognized by the ocularist to understand the elements to be evaluated in the fellow eye. -

Rendering of Eyes for Eye-Shape Registration and Gaze Estimation
Rendering of Eyes for Eye-Shape Registration and Gaze Estimation Erroll Wood1, Tadas Baltrusaitisˇ 1, Xucong Zhang2, Yusuke Sugano2, Peter Robinson1, and Andreas Bulling2 1University of Cambridge, United Kingdom feww23,tb346,[email protected] 2Max Planck Institute for Informatics, Germany fxczhang,sugano,[email protected] Abstract—Images of the eye are key in several computer vision Rendered training images Eye-shape registration problems, such as shape registration and gaze estimation. Recent large-scale supervised methods for these problems require time- consuming data collection and manual annotation, which can be unreliable. We propose synthesizing perfectly labelled photo- realistic training data in a fraction of the time. We used computer graphics techniques to build a collection of dynamic eye-region Gaze estimation models from head scan geometry. These were randomly posed to synthesize close-up eye images for a wide range of head poses, gaze directions, and illumination conditions. We used our model’s controllability to verify the importance of realistic illumination and shape variations in eye-region training data. pitch = 15°, yaw = 9° Finally, we demonstrate the benefits of our synthesized training data (SynthesEyes) by out-performing state-of-the-art methods Fig. 1: We render a large number of photorealistic images of eyes for eye-shape registration as well as cross-dataset appearance- using a dynamic eye region model. These are used as training data based gaze estimation in the wild. for eye-shape registration and appearance-based gaze estimation. I. INTRODUCTION The eyes and their movements convey our attention and it undergoes with facial motion and eyeball rotation, and the play a role in communicating social and emotional information complex material structure of the eyeball itself. -

The Horizontal Raphe of the Human Retina and Its Watershed Zones
vision Review The Horizontal Raphe of the Human Retina and its Watershed Zones Christian Albrecht May * and Paul Rutkowski Department of Anatomy, Medical Faculty Carl Gustav Carus, TU Dresden, 74, 01307 Dresden, Germany; [email protected] * Correspondence: [email protected] Received: 24 September 2019; Accepted: 6 November 2019; Published: 8 November 2019 Abstract: The horizontal raphe (HR) as a demarcation line dividing the retina and choroid into separate vascular hemispheres is well established, but its development has never been discussed in the context of new findings of the last decades. Although factors for axon guidance are established (e.g., slit-robo pathway, ephrin-protein-receptor pathway) they do not explain HR formation. Early morphological organization, too, fails to establish a HR. The development of the HR is most likely induced by the long posterior ciliary arteries which form a horizontal line prior to retinal organization. The maintenance might then be supported by several biochemical factors. The circulation separate superior and inferior vascular hemispheres communicates across the HR only through their anastomosing capillary beds resulting in watershed zones on either side of the HR. Visual field changes along the HR could clearly be demonstrated in vascular occlusive diseases affecting the optic nerve head, the retina or the choroid. The watershed zone of the HR is ideally protective for central visual acuity in vascular occlusive diseases but can lead to distinct pathological features. Keywords: anatomy; choroid; development; human; retina; vasculature 1. Introduction The horizontal raphe (HR) was first described in the early 1800s as a horizontal demarcation line that extends from the macula to the temporal Ora dividing the temporal retinal nerve fiber layer into a superior and inferior half [1].