Biomechanical Modelling of the Human Eye
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

CHQ-GDL-01074 Acute Management of Open Globe Injuries
Acute management of Open Globe Injuries Document ID CHQ-GDL-01074 Version no. 2.0 Approval date 14/05/2020 Executive sponsor Executive Director Medical Services Effective date 14/05/2020 Author/custodian Director Infection Management and Prevention service, Review date 14/05/2022 Immunology and Rheumatology Supersedes 1.0 Applicable to All Children’s Health Queensland (CHQ) staff Authorisation Executive Director Clinical Services (QCH) Purpose This evidence-based guideline provides clinical practice advice for clinicians for the acute management of children with open globe injuries. A paediatric ophthalmology team must be actively involved in the management of all patients presenting with this condition. Scope This guideline applies to all Children’s Health Queensland (CHQ) Staff treating a child presenting for the management of open globe injury. Related documents • CHQ-GDL-01202 CHQ Paediatric Antibiocard: Empirical Antibiotic Guidelines • CHQ-PROC-01035 Antimicrobial Restrictions • CHQ Antimicrobial Restriction list • CHQ-GDL-01023 Tetanus Prophylaxis in Wound Management CHQ-GDL-01074- Acute management of Open Globe Injuries - 1 - Guideline Introduction Ocular trauma is an important cause of eye morbidity and is a leading cause of non-congenital mono-ocular blindness among children.1 A quarter of a million children present each year with serious ocular trauma. The vast majority of these are preventable.2 Open globe injuries are injuries where the cornea and/or sclera are breached and there is a full-thickness wound of the eye wall.3 It can be further delineated into globe rupture from blunt trauma and lacerations from sharp objects. When a large blunt object impacts onto the eye, there is an instant increase in intraocular pressure and the eye wall yields at its weakest point leading to tissue prolapse.4 Open globe lacerations are caused by sharp objects or projectiles and subdivided into either penetrating or perforating injuries. -

Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts After Long-Duration Space Flight
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 10-2011 Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight Thomas H. Mader Alaska Native Medical Center, [email protected] C. Robert Gibson Coastal Eye Associates Anastas F. Pass University of Houston Larry A. Kramer University of Texas Health Science Center Andrew G. Lee The Methodist Hospital See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/nasapub Part of the Physical Sciences and Mathematics Commons Mader, Thomas H.; Gibson, C. Robert; Pass, Anastas F.; Kramer, Larry A.; Lee, Andrew G.; Fogarty, Jennifer; Tarver, William J.; Dervay, Joseph P.; Hamilton, Douglas R.; Sargsyan, Ashot; Phillips, John L.; Tran, Duc; Lipsky, William; Choi, Jung; Stern, Claudia; Kuyumjian, Raffi; andolk, P James D., "Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight" (2011). NASA Publications. 69. https://digitalcommons.unl.edu/nasapub/69 This Article is brought to you for free and open access by the National Aeronautics and Space Administration at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in NASA Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Thomas H. Mader, C. Robert Gibson, Anastas F. Pass, Larry A. -

Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception
The Journal of Neuroscience, January 28, 2015 • 35(4):1493–1504 • 1493 Behavioral/Cognitive Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception Elena Pretegiani,1,2 Corina Astefanoaei,3 XPierre M. Daye,1,4 Edmond J. FitzGibbon,1 Dorina-Emilia Creanga,3 Alessandra Rufa,2 and XLance M. Optican1 1Laboratory of Sensorimotor Research, NEI, NIH, DHHS, Bethesda, Maryland, 20892-4435, 2EVA-Laboratory, University of Siena, 53100 Siena, Italy, 3Alexandru Ioan Cuza University, Physics Faculty, 700506 Iasi, Romania, and 4Institut du cerveau et de la moelle´pinie e `re (ICM), INSERM UMRS 975, 75013 Paris, France We move our eyes to explore the world, but visual areas determining where to look next (action) are different from those determining what we are seeing (perception). Whether, or how, action and perception are temporally coordinated is not known. The preparation time course of an action (e.g., a saccade) has been widely studied with the gap/overlap paradigm with temporal asynchronies (TA) between peripheral target onset and fixation point offset (gap, synchronous, or overlap). However, whether the subjects perceive the gap or overlap, and when they perceive it, has not been studied. We adapted the gap/overlap paradigm to study the temporal coupling of action and perception. Human subjects made saccades to targets with different TAs with respect to fixation point offset and reported whether they perceived the stimuli as separated by a gap or overlapped in time. Both saccadic and perceptual report reaction times changed in the same way as a function of TA. The TA dependencies of the time change for action and perception were very similar, suggesting a common neural substrate. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -
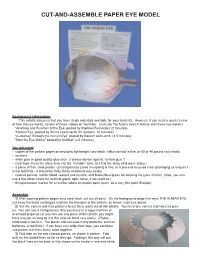
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

Trauma Suturing Techniques 7 Marian S
Chapter 7 Trauma Suturing Techniques 7 Marian S. Macsai and Bruno Machado Fontes Key Points 7.1 Introduction • Assess the presence of life-threatening inju- ries. Ocular trauma is an important cause of unilateral vi- • Vision at the time of presentation and the sion loss worldwide, especially in young people, and presence or absence of aff erent pupillary de- surgical repair is almost always challenging [1–7]. A fect are important prognostic factors in the patient with an eye injury may need immediate inter- Ocular Trauma Classifi cation System [1]. vention, and all ophthalmologists who cover emergen- • Surgical goals include: cy patients must have the knowledge and skills to deal – Watertight wound closure with diffi cult and complex surgeries, as these initial ac- – Restoration of normal anatomic relation- tions and interventions may be determinants for the ships fi nal visual prognosis [7–15]. One must keep in the – Restoration of optimal visual function mind that the result of the fi rst surgery will determine – Prevention of possible future complica- the need for future reconstruction. tions Th e epidemiology of ocular trauma varies according • Surgical indications: to the region studied. In the World Trade Center disas- – Any perforating injury ter, ocular trauma was found to be the second most – Any wound with tissue loss common type of injury among survivors [16]. Th e – Any clinical suspicion of globe rupture re- most common causes of eye injuries include automo- quires exploration and possible repair tive, domestic, and occupational accidents, together • Instrumentation: with violence. Risk factors most commonly described – Complete ophthalmic microsurgical tray for eye injuries are male gender (approximately 80% of – Phacoemulsifi cation, vitrectomy and irri- open-globe injuries), race (Hispanics and African- gation and aspiration machines Americans have higher risk), professional activity (e. -
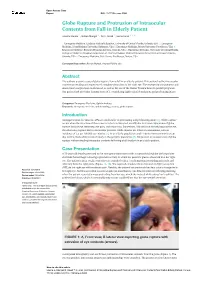
Globe Rupture and Protrusion of Intraocular Contents from Fall in Elderly Patient
Open Access Case Report DOI: 10.7759/cureus.5988 Globe Rupture and Protrusion of Intraocular Contents from Fall in Elderly Patient Andrew Hanna 1 , Rohan Mangal 2 , Tej G. Stead 3 , Latha Ganti 4, 5, 6 1. Emergency Medicine, Graduate Medical Education, University of Central Florida, Orlando, USA 2. Emergency Medicine, Johns Hopkins University, Baltimore, USA 3. Emergency Medicine, Brown University, Providence, USA 4. Emergency Medicine, Envision Physician Services, Orlando, USA 5. Emergency Medicine, University of Central Florida College of Medicine / Hospital Corporation of America Graduate Medical Education Consortium of Greater Orlando, Orlando, USA 6. Emergency Medicine, Polk County Fire Rescue, Bartow, USA Corresponding author: Rohan Mangal, [email protected] Abstract The authors present a case of globe rupture from a fall in an elderly patient. This patient had her intraocular contents protruding and experienced complete vision loss in her right eye. The emergency management and downstream surgical care is discussed, as well as the use of the Ocular Trauma Score to predict prognosis. Our patient had an Ocular Trauma Score of 1, considering right retinal detachment and perforating injury. Categories: Emergency Medicine, Ophthalmology Keywords: emergency medicine, ophthalmology, trauma, globe rupture Introduction Amongst serious eye injuries, 40% are attributable to penetrating and perforating injury [1]. Globe rupture occurs when the structure of the cornea or sclera is disrupted, usually due to trauma. Symptoms of globe rupture include eye deformity, eye pain, and vision loss. Sometimes, if blunt force directly impacts the eye, the sclera may rupture due to intraocular pressure. Globe injuries are relatively uncommon, with an incidence of 3.5 per 100,000 eye injuries [2]. -

The Evolution of Human Intelligence and the Coefficient of Additive Genetic Variance in Human Brain Size ⁎ Geoffrey F
Intelligence 35 (2007) 97–114 The evolution of human intelligence and the coefficient of additive genetic variance in human brain size ⁎ Geoffrey F. Miller a, , Lars Penke b a University of New Mexico, USA b Institut für Psychologie, Humboldt-Universität zu Berlin, Germany Received 3 November 2005; received in revised form 17 August 2006; accepted 18 August 2006 Available online 12 October 2006 Abstract Most theories of human mental evolution assume that selection favored higher intelligence and larger brains, which should have reduced genetic variance in both. However, adult human intelligence remains highly heritable, and is genetically correlated with brain size. This conflict might be resolved by estimating the coefficient of additive genetic variance (CVA) in human brain size, since CVAs are widely used in evolutionary genetics as indexes of recent selection. Here we calculate for the first time that this CVA is about 7.8, based on data from 19 recent MRI studies of adult human brain size in vivo: 11 studies on brain size means and standard deviations, and 8 studies on brain size heritabilities. This CVA appears lower than that for any other human organ volume or life-history trait, suggesting that the brain has been under strong stabilizing (average-is-better) selection. This result is hard to reconcile with most current theories of human mental evolution, which emphasize directional (more-is-better) selection for higher intelligence and larger brains. Either these theories are all wrong, or CVAs are not as evolutionarily informative as most evolutionary geneticists believe, or, as we suggest, brain size is not a very good index for understanding the evolutionary genetics of human intelligence. -

Arteriovenous Dissection in a Living Human
Vienna, Austria, 1990. Dordrecht, Holland: Klu- Table 2. Ultrasonographic Findings of 25 Well-Documented Patients wer Academic Publishers; 1993:307-311. With Cavitary Melanoma of the Uvea in English Literature 9. Frazier-Byrne S, Green RL. Intraocular tumors. In: Frazier-Byrne S, Green RL, eds. Ultrasound of the Eye and Orbit. 2nd ed. St Louis, Mo: Ultrasonographic Findings Mosby; 2002:115-190. 10. Scott CT, Holland GN, Glasgow BJ. Cavita- Solid % Mass tion in ciliary body melanoma. Am J Ophthalmol. Component Loculation Echoes in Septa in Thickness Occupied 1997;123:269-271. Source Present on USG Cavitation Cavitation by Cavity 11. Cohen PR, Rapini RP. Nevus with cyst: a re- port of 93 cases. Am J Dermatopathol. 1993; Kennedy5 NA NA NA NA NA 15:229-234. NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA Reese6 NA NA NA NA NA Arteriovenous Dissection Zakka et al7 ϩ Unilocular ϩ −NA in a Living Human Eye: Stone and Shapiro4 ϩ Unilocular ϩ −65 ϩ Unilocular ϩ −75 Clinicopathologic − Unilocular ϩ −60 Correlation ϩ Unilocular − − 30 ϩ Multilocular ϩϩ 40 Fledelius et al8 − Unilocular − − 75 Although the visual results after ar- Scott et al10 − Multilocular ϩϩ NA teriovenous dissection (AVD) seem 1,2 Lois et al2 − Unilocular ϩ −79 encouraging, its effectiveness has ϩ Unilocular − − 59 not been proved in a controlled, pro- ϩ Multilocular − ϩ 31 spective clinical trial. The role of sur- ϩ Multilocular ϩϩ 59 gical decompression itself remains ϩ − Unilocular −64 unclear,3 and little is known about − Multilocular ϩϩ 62 ϩ Unilocular ϩ −55 surgically induced nerve fiber de- ϩ Multilocular ϩϩ 38 fects. -

Root Eye Dictionary a "Layman's Explanation" of the Eye and Common Eye Problems
Welcome! This is the free PDF version of this book. Feel free to share and e-mail it to your friends. If you find this book useful, please support this project by buying the printed version at Amazon.com. Here is the link: http://www.rooteyedictionary.com/printversion Timothy Root, M.D. Root Eye Dictionary A "Layman's Explanation" of the eye and common eye problems Written and Illustrated by Timothy Root, M.D. www.RootEyeDictionary.com 1 Contents: Introduction The Dictionary, A-Z Extra Stuff - Abbreviations - Other Books by Dr. Root 2 Intro 3 INTRODUCTION Greetings and welcome to the Root Eye Dictionary. Inside these pages you will find an alphabetical listing of common eye diseases and visual problems I treat on a day-to-day basis. Ophthalmology is a field riddled with confusing concepts and nomenclature, so I figured a layman's dictionary might help you "decode" the medical jargon. Hopefully, this explanatory approach helps remove some of the mystery behind eye disease. With this book, you should be able to: 1. Look up any eye "diagnosis" you or your family has been given 2. Know why you are getting eye "tests" 3. Look up the ingredients of your eye drops. As you read any particular topic, you will see that some words are underlined. An underlined word means that I've written another entry for that particular topic. You can flip to that section if you'd like further explanation, though I've attempted to make each entry understandable on its own merit. I'm hoping this approach allows you to learn more about the eye without getting bogged down with minutia .. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst.