Reticulate Pigmentary Disorders Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

A Mutation in Lamin A/C Gene Previously Known to Cause Emery
ical C lin as C e f R o l e a p n o r r t u s o J Journal of Clinical Case Reports Chalissery et al., J Clin Case Rep 2016, 6:4 ISSN: 2165-7920 DOI: 10.4172/2165-7920.1000770 Case Report Open Access A Mutation in Lamin A/C Gene Previously Known to Cause Emery- Driefuss Muscular Dystrophy Causing A Phenotype of Limb Girdle Muscular Dystrophy Type 1B Albi J Chalissery1*, Tudor Munteanu1, Yvonne Langan2, Francesca Brett2 and Janice Redmond1 1Department of Neurology, St James’s Hospital, Ireland 2Department of Neurophysiology, St James’s Hospital, Ireland 3Department of Neuropathology, Beaumont Hospital, Dublin, Ireland *Corresponding author: Albi J Chalissery, Department of Neurology, St James’s Hospital, James’s Street, Dublin 8, Ireland, Tel +353 1 410 3000; E-mail: [email protected] Rec date: Feb 19, 2016; Acc date: Apr 13, 2016; Pub date: Apr 18, 2016 Copyright: © 2016 Chalissery AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Mutations in the lamin protein(found in the nuclear envelope) known to cause different allelic disorders including limb girdle muscular dystrophies (LGMD) and Emery-Dreifuss muscular dystrophy (EDMD). LGMDs are a heterogeneous group of disorders with progressive proximal muscle weakness in an autosomal inheritance pattern. LGMD type 1B is a disorder secondary to a mutation in the gene encoding Lamin A/C protein in the nuclear envelope. -

Frequency of Different Types of Facial Melanoses Referring to the Department of Dermatology and Venereology, Nepal Medical Colle
Amatya et al. BMC Dermatology (2020) 20:4 https://doi.org/10.1186/s12895-020-00100-3 RESEARCH ARTICLE Open Access Frequency of different types of facial melanoses referring to the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital in 2019, and assessment of their effect on health-related quality of life Bibush Amatya* , Anil Kumar Jha and Shristi Shrestha Abstract Background: Abnormalities of facial pigmentation, or facial melanoses, are a common presenting complaint in Nepal and are the result of a diverse range of conditions. Objectives: The objective of this study was to determine the frequency, underlying cause and impact on quality of life of facial pigmentary disorders among patients visiting the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital (NMCTH) over the course of one year. Methods: This was a cross-sectional study conducted at the Department of Dermatology and Venereology, NMCT H. We recruited patients with facial melanoses above 16 years of age who presented to the outpatient department. Clinical and demographic data were collected and all the enrolled participants completed the validated Nepali version of the Dermatology Life Quality Index (DLQI). Results: Between January 5, 2019 to January 4, 2020, a total of 485 patients were recruited in the study. The most common diagnoses were melasma (166 patients) and post acne hyperpigmentation (71 patients). Quality of life impairment was highest in patients having melasma with steroid induced rosacea-like dermatitis (DLQI = 13.54 ± 1.30), while it was lowest in participants with ephelides (2.45 ± 1.23). Conclusion: Facial melanoses are a common presenting complaint and lead to substantial impacts on quality of life. -

Melasma on the Nape of the Neck in a Man
Letters to the Editor 181 Melasma on the Nape of the Neck in a Man Ann A. Lonsdale-Eccles and J. A. A. Langtry Sunderland Royal Hospital, Kayll Road, Sunderland SR4 7TP, UK. E-mail: [email protected] Accepted July 19, 2004. Sir, sunlight and photosensitizing agents may be more We report a 47-year-old man with light brown macular relevant. pigmentation on the nape of his neck (Fig. 1). It was The differential diagnosis for pigmentation at this site asymptomatic and had developed gradually over 2 years. includes Riehl’s melanosis, Berloque dermatitis and He worked outdoors as a pipe fitter on an oilrig module; poikiloderma of Civatte. Riehl’s melanosis typically however, he denied exposure at this site because he involves the face with a brownish-grey pigmentation; always wore a shirt with a collar that covered the biopsy might be expected to show interface change and affected area. However, on further questioning it liquefaction basal cell degeneration with a moderate transpired that he spent most of the day with his head lymphohistiocytic infiltrate, melanophages and pigmen- bent forward. This reproducibly exposed the area of tary incontinence in the upper dermis. It is usually pigmentation with a sharp cut off inferiorly at the level associated with cosmetic use and may be considered of his collar. He used various shampoos, aftershaves and synonymous with pigmented allergic contact dermatitis shower gels, but none was applied directly to that area. of the face (6, 7). Berloque dermatitis is considered to be His skin was otherwise normal and there was no family caused by a photoirritant reaction to bergapentin; it history of abnormal pigmentation. -

Dyskeratosis Congenita Precision Panel Overview Indications Clinical
Dyskeratosis Congenita Precision Panel Overview Dyskeratosis Congenita (DKC) is a rare, progressive bone marrow failure syndrome characterized by reticulated skin hyperpigmentation, nail dystrophy and oral leukoplakia. Patients usually present with symptoms of skin hyperpigmentation and nail changes during the first decade of life. It is caused by germline mutations in genes regulating telomere maintenance, resulting in very short telomeres. DKC is a genetically heterogeneous with X-linked recessive form being the most common, autosomal dominant and autosomal recessive subtypes based on different patters of inheritance. Early mortality is associated with bone marrow failure, infections, lung and pulmonary complications as well as malignancy. The Igenomix Dyskeratosis Congenita Precision Panel can be used for an accurate and directed diagnosis as well as differential diagnosis of reticulate pigmentary disorders ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved. Indications The Igenomix Dyskeratosis Congenita Precision Panel is used for patients with a clinical diagnosis or suspicion with or without the following symptoms: ‐ Abnormal skin pigmentation (tan-to-gray hyperpigmented or hypopigmented macules and patches) ‐ Nail dystrophy ‐ Skin atrophy and telangiectasia ‐ Alopecia of the skin, eyebrows and eyelashes ‐ Mucosal leukoplakia ‐ Bone marrow failure ‐ Dental manifestations Clinical Utility The clinical utility of this panel is: ‐ The genetic and molecular confirmation for an accurate clinical diagnosis of a symptomatic patient. 1 ‐ Early initiation of treatment involving a multidisciplinary team in the form of hematopoietic stem cell transplantation as well as medical care to prevent complications and early surveillance of malignancy. -

An Unusual Presentation of a Unilateral Asymptomatic Riehl's
ts & C por a e se R S l Michael, Med Rep Case Stud 2018, 3:1 t a u c d i i DOI: 10.4172/2572-5130.1000152 d e s e M + Medical Reports and Case Studies ISSN: 2572-5130 Case Report Open Access An Unusual Presentation of a Unilateral Asymptomatic Riehl’s Melanosis in a 45 Year Old Male Chan Kam Tim Michael* Department of Dermatology, Hong Kong Academy of Medicine, Hong Kong *Corresponding author: Chan Kam Tim Michael, Department of Dermatology, Hong Kong Academy of Medicine, Hong Kong, Tel: +85221282129; E-mail: [email protected] Received Date: Feb 12, 2018; Accepted Date: Mar 12, 2018; Published Date: Mar 21, 2018 Copyright: © 2018 Michael CKT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Introduction but of post-inflammatory hyperpigmentation, Acquired unilateral Nevus (Hori’s Nevus), Riehl’s melanosis, Drug-induced Pigmented contact dermatitis (PCD), also known as Riehl’s hyperpigmentation, Lichenoid dermatitis; as well as Melasma and melanosis, is a rare facial hyperpigmentation usually secondary to Ochronosis. cosmetics. There are few documented reports in the literature, and many cases without proven diagnosis may have been treated with pigment lasers, especially in beauty parlour settings. We report a case referred from a private practitioner who has a special interest in dermatology. The patient was diagnosed subsequently as having Riehl’s melanosis and treated with non-tyrosinase inhibitor bleaching agents, sun avoidance and mandatory abstinence from over-the-counter cosmetic products. -
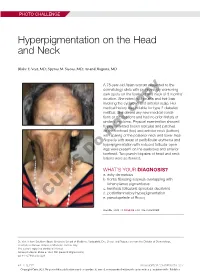
Hyperpigmentation on the Head and Neck
PHOTO CHALLENGE Hyperpigmentation on the Head and Neck Blake E. Vest, MD; Spyros M. Siscos, MD; Anand Rajpara, MD A 78-year-old Asian woman presented to the dermatology clinic with progressively worsening dark spots on the forehead and neck of 3 months’ duration. She noted mild pruritis and hair loss involving the eyebrows and anterior scalp. Her medical history was notable for type 2 diabetes mellitus. She deniedcopy any new medical condi- tions or medications and had no prior history of similar symptoms. Physical examination showed hyperpigmented brown macules and patches on the forehead (top) and anterior neck (bottom) withnot sparing of the posterior neck and lower face. Alopecia with areas of perifollicular erythema and hyperpigmentation with reduced follicular open- ings were present on the eyebrows and anterior Doforehead. Two punch biopsies of head and neck lesions were performed. WHAT’S YOUR DIAGNOSIS? a. ashy dermatosis b. frontal fibrosing alopecia overlapping with lichen planus pigmentosus c. keratosis follicularis spinulosa decalvans d. postinflammatory hyperpigmentation CUTIS e. pseudopelade of Brocq PLEASE TURN TO PAGE E3 FOR THE DIAGNOSIS Dr. Vest is from Southern Illinois University School of Medicine, Springfield. Drs. Siscos and Rajpara are from the Division of Dermatology, University of Kansas School of Medicine, Kansas City. The authors report no conflict of interest. Correspondence: Blake E. Vest, MD ([email protected]). doi:10.12788/cutis.0226 E2 I CUTIS® WWW.MDEDGE.COM/DERMATOLOGY Copyright Cutis 2021. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus icroscopic examination revealed focal dermal of the skin have been reported, including the entire pigmentation, papillary fibrosis, and epidermal scalp, eyebrows, and eyelashes. -

The Genetics and Clinical Manifestations of Telomere Biology Disorders Sharon A
REVIEW The genetics and clinical manifestations of telomere biology disorders Sharon A. Savage, MD1, and Alison A. Bertuch, MD, PhD2 3 Abstract: Telomere biology disorders are a complex set of illnesses meric sequence is lost with each round of DNA replication. defined by the presence of very short telomeres. Individuals with classic Consequently, telomeres shorten with aging. In peripheral dyskeratosis congenita have the most severe phenotype, characterized blood leukocytes, the cells most extensively studied, the rate 4 by the triad of nail dystrophy, abnormal skin pigmentation, and oral of attrition is greatest during the first year of life. Thereafter, leukoplakia. More significantly, these individuals are at very high risk telomeres shorten more gradually. When the extent of telo- of bone marrow failure, cancer, and pulmonary fibrosis. A mutation in meric DNA loss exceeds a critical threshold, a robust anti- one of six different telomere biology genes can be identified in 50–60% proliferative signal is triggered, leading to cellular senes- of these individuals. DKC1, TERC, TERT, NOP10, and NHP2 encode cence or apoptosis. Thus, telomere attrition is thought to 1 components of telomerase or a telomerase-associated factor and TINF2, contribute to aging phenotypes. 5 a telomeric protein. Progressively shorter telomeres are inherited from With the 1985 discovery of telomerase, the enzyme that ex- generation to generation in autosomal dominant dyskeratosis congenita, tends telomeric nucleotide repeats, there has been rapid progress resulting in disease anticipation. Up to 10% of individuals with apparently both in our understanding of basic telomere biology and the con- acquired aplastic anemia or idiopathic pulmonary fibrosis also have short nection of telomere biology to human disease. -

Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Prenatalscreen® Standard Technical Report
About PrenatalScreen® Prenatal Test PrenatalScreen® Prenatal Test is a genetic test that analyses fetal DNA, obtained from CVS or amniotic fluid following an invasive prenatal diagnosis, to screen for monogenic disorders in the fetus. Using the latest technologies, including Next Generation Sequencing (NGS), PrenatalScreen® Prenatal Test screen 744 genes for mutations causing over 1.000 severe genetic disorders in the fetus. PrenatalScreen® Prenatal Test allows for a comprehensive care and enables patients to make more informed reproductive decisions. Offering PrenatalScreen® Prenatal Test to a patient during pregnancy allows her to gain more knowledge about the potential to pass along a condition to the fetus. Aim of the test PrenatalScreen® Prenatal Test analyses DNA extracted from fetal cells in the amniotic fluid, collected through amniocentesis, or in the chorionic villi through villocentesis (CVS). The aim of this diagnositc test is to assess severe genetic diseases in the fetus, including the most common diseases in the European population. Genes listed in Table 1 were selected according to the incidence in the population of the disease caused by mutations in such genes, the severity of the clinical phenotype at birth and the importance of the related pathogenetic picture, in accordance with the indications of the American College of Medical Genetics (ACMG)(Grody et al., Genet Med 2013:15:482–483). PrenatalScreen®: Indication for testing PrenatalScreen® Prenatal Test is intended for patients who meet any of the following criteria: • Personal/familial anamnesis of hereditary genetic diseases; • For expectant mothers wishing to reduce the risk of a genetic diseases in the fetus; • For natural or in vitro fertilization (IVF)-derived pregnancies: • For couples using heterologus IVF procedures (egg/sperm donors). -

Blueprint Genetics Comprehensive Skeletal Dysplasias and Disorders
Comprehensive Skeletal Dysplasias and Disorders Panel Test code: MA3301 Is a 251 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of disorders involving the skeletal system. About Comprehensive Skeletal Dysplasias and Disorders This panel covers a broad spectrum of skeletal disorders including common and rare skeletal dysplasias (eg. achondroplasia, COL2A1 related dysplasias, diastrophic dysplasia, various types of spondylo-metaphyseal dysplasias), various ciliopathies with skeletal involvement (eg. short rib-polydactylies, asphyxiating thoracic dysplasia dysplasias and Ellis-van Creveld syndrome), various subtypes of osteogenesis imperfecta, campomelic dysplasia, slender bone dysplasias, dysplasias with multiple joint dislocations, chondrodysplasia punctata group of disorders, neonatal osteosclerotic dysplasias, osteopetrosis and related disorders, abnormal mineralization group of disorders (eg hypopohosphatasia), osteolysis group of disorders, disorders with disorganized development of skeletal components, overgrowth syndromes with skeletal involvement, craniosynostosis syndromes, dysostoses with predominant craniofacial involvement, dysostoses with predominant vertebral involvement, patellar dysostoses, brachydactylies, some disorders with limb hypoplasia-reduction defects, ectrodactyly with and without other manifestations, polydactyly-syndactyly-triphalangism group of disorders, and disorders with defects in joint formation and synostoses. Availability 4 weeks Gene Set Description