Species Distribution Modeling and Assessment of Environmental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Particulars of Some Temples of Kerala Contents Particulars of Some
Particulars of some temples of Kerala Contents Particulars of some temples of Kerala .............................................. 1 Introduction ............................................................................................... 9 Temples of Kerala ................................................................................. 10 Temples of Kerala- an over view .................................................... 16 1. Achan Koil Dharma Sastha ...................................................... 23 2. Alathiyur Perumthiri(Hanuman) koil ................................. 24 3. Randu Moorthi temple of Alathur......................................... 27 4. Ambalappuzha Krishnan temple ........................................... 28 5. Amedha Saptha Mathruka Temple ....................................... 31 6. Ananteswar temple of Manjeswar ........................................ 35 7. Anchumana temple , Padivattam, Edapalli....................... 36 8. Aranmula Parthasarathy Temple ......................................... 38 9. Arathil Bhagawathi temple ..................................................... 41 10. Arpuda Narayana temple, Thirukodithaanam ................. 45 11. Aryankavu Dharma Sastha ...................................................... 47 12. Athingal Bhairavi temple ......................................................... 48 13. Attukkal BHagawathy Kshethram, Trivandrum ............. 50 14. Ayilur Akhileswaran (Shiva) and Sri Krishna temples ........................................................................................................... -

Accused Persons Arrested in Kollam Rural District from 17.11.2019To23.11.2019
Accused Persons arrested in Kollam Rural district from 17.11.2019to23.11.2019 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 19.11.201 Cr. 1489/19 Muhammad Muhammad Manzil Abdul Vahid, JFMC I 1 Shajahan 20 Yeroor 9 08.15 U/S 457, 380 yeroor Sahad Veedu, Pathady SI Yeroor Punalur Hrs & 34 IPC 19.11.201 Vadakkumkara puthen Cr. 1489/19 Abdul Vahid, 2 9 08.15 SI Yeroor veedu, Kanjuvayal, Hrs U/S 457, 380 & JFMC I Hussain Sunu 19 Pathady Yeroor 34 IPC yeroor Punalur 19.11.201 Cr. 1489/19 Abdul Vahid, 3 Rafeeka Manzil, 9 08.15 U/S 457, 380 & JFMC I SI Yeroor Al Ameen Anzar, 19 athady, Yeroor Yeroor Hrs 34 IPC yeroor Punalur Shiyas Manzil, Cr. 1489/19 Subin 4 Randekkarmukk, 17.11.2019 U/S 457, 380 & Thankachan, SI, JFMC I Shiyas Shereef 19 Yeroor Yeroor 12.00 Hrs 34 IPC yeroor Yeroor Punalur Cr. 1489/19 Subin 5 Thembamvila veedu, 17.11.2019, U/S 457, 380 & Thankachan, SI, JFMC I Noufal Noushad 20 Pathady, Yeroor Yeroor 12.00 Hrs 34 IPC yeroor Yeroor Punalur 19.11.201 Cr. 1489/19 Abdul Vahid, 6 Plavila puthen veedu, 9 08.15 U/S 457, 380 & JFMC I SI Yeroor AlMubarak Sainudeen 23 Kanjuvayal, Pathady Yeroor Hrs 34 IPC yeroor Punalur 19.11.201 Cr. -

KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with Financial Assistance from the World Bank
KERALA SOLID WASTE MANAGEMENT Public Disclosure Authorized PROJECT (KSWMP) INTRODUCTION AND STRATEGIC ENVIROMENTAL ASSESSMENT OF WASTE Public Disclosure Authorized MANAGEMENT SECTOR IN KERALA VOLUME I JUNE 2020 Public Disclosure Authorized Prepared by SUCHITWA MISSION Public Disclosure Authorized GOVERNMENT OF KERALA Contents 1 This is the STRATEGIC ENVIRONMENTAL ASSESSMENT OF WASTE MANAGEMENT SECTOR IN KERALA AND ENVIRONMENTAL AND SOCIAL MANAGEMENT FRAMEWORK for the KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with financial assistance from the World Bank. This is hereby disclosed for comments/suggestions of the public/stakeholders. Send your comments/suggestions to SUCHITWA MISSION, Swaraj Bhavan, Base Floor (-1), Nanthancodu, Kowdiar, Thiruvananthapuram-695003, Kerala, India or email: [email protected] Contents 2 Table of Contents CHAPTER 1. INTRODUCTION TO THE PROJECT .................................................. 1 1.1 Program Description ................................................................................. 1 1.1.1 Proposed Project Components ..................................................................... 1 1.1.2 Environmental Characteristics of the Project Location............................... 2 1.2 Need for an Environmental Management Framework ........................... 3 1.3 Overview of the Environmental Assessment and Framework ............. 3 1.3.1 Purpose of the SEA and ESMF ...................................................................... 3 1.3.2 The ESMF process ........................................................................................ -
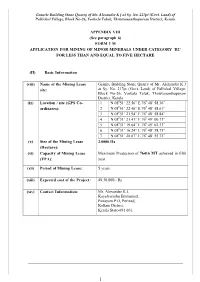
Form 1 M Application for Mining of Minor Minerals Under Category ‘B2’ for Less Than and Equal to Five Hectare
Granite Building Stone Quarry of Mr. Alexander K J at Sy. No. 217pt (Govt. Land) of Pallickal Village, Block No-26, Varkala Taluk, Thiruvananthapuram District, Kerala APPENDIX VIII (See paragraph 6) FORM 1 M APPLICATION FOR MINING OF MINOR MINERALS UNDER CATEGORY ‘B2’ FOR LESS THAN AND EQUAL TO FIVE HECTARE (II) Basic Information (viii) Name of the Mining Lease Granite Building Stone Quarry of Mr. Alexander K J site: at Sy. No. 217pt (Govt. Land) of Pallickal Village, Block No-26, Varkala Taluk, Thiruvananthapuram District, Kerala 0 0 (ix) Location / site (GPS Co- 1 N 08 51’ 22.56” E 76 48’ 58.36” 0 0 ordinates): 2 N 08 51’ 22.46” E 76 48’ 58.61” 0 0 3 N 08 51’ 21.94” E 76 48’ 58.84” 0 0 4 N 08 51’ 21.41” E 76 49’ 00.73” 0 0 5 N 08 51’ 19.64” E 76 49’ 02.33” 0 0 6 N 08 51’ 16.24” E 76 48’ 58.75” 0 0 7 N 08 51’ 20.07” E 76 48’ 55.72” (x) Size of the Mining Lease 2.0000 Ha (Hectare): (xi) Capacity of Mining Lease Maximum Production of 76416 MT achieved in fifth (TPA): year. (xii) Period of Mining Lease: 5 years (xiii) Expected cost of the Project: 49,30,000/- Rs (xiv) Contact Information: Mr. Alexander K J, Kayalvarathu Emmanuel, Panayam P.O, Perinad, Kollam District, Kerala State-691 601. 1 Granite Building Stone Quarry of Mr. Alexander K J at Sy. No. 217pt (Govt. -

Economic Analysis of Sand Mining in Bharathapuzha River, Kerala, India
ISSN: 2349-5677 Volume 1, Issue 4, September 2014 Economic Analysis Of Sand Mining In Bharathapuzha River, Kerala, India Moinak Maiti MBA Banking Technology, Pondicherry Central University, Pondicherry, India [email protected] Abstract Globalisation of the developing countries like India demands for the more natural resources product for rapid development. The rapid infrastructure development and urbanisation leads to the mismatch in the demand and supply. The scarcity of resources leads to the illegal activity and degradation of the environment. This paper addresses the issues relating to sand mining in the BharathapuzhaRiver in Kerala India, its economic analysis and its consequences. Finally attempts to bring the attention of the international community for sustainable growth. Index terms: Sand mining, economic analysis, sustainable growth Introduction The Bharathapuzhariver in the Indian state known to be the Nila of Kerala. The Bharathapuzha River covers 155 KM in Kerala. The details course of the river has been shown in the figure no. 1. During of its course it covers the areas like South Chittur, kannadi Bridge, kalpathiPuzha, Mankara, Cheerakuzhi, Pattambi, thrithala, ThuthaPuzha, Kuttipuram and Ponnani sea level etc. In its upper course River carries large size rocks at high velocity due to higher steep. However, this inclination decreases from Pattambi to Kuttipuram Region where sand is deposited by the river. Sand mining is primarily carried out in this region. The river has over dammed in its course, mainly its water used for the irrigation purpose. The rapid globalisation and development leads to the scarcity of the natural resources like sand. As sand are the primary integrands for the construction projects. -

Pathanamthitta
Census of India 2011 KERALA PART XII-A SERIES-33 DISTRICT CENSUS HANDBOOK PATHANAMTHITTA VILLAGE AND TOWN DIRECTORY DIRECTORATE OF CENSUS OPERATIONS KERALA 2 CENSUS OF INDIA 2011 KERALA SERIES-33 PART XII-A DISTRICT CENSUS HANDBOOK Village and Town Directory PATHANAMTHITTA Directorate of Census Operations, Kerala 3 MOTIF Sabarimala Sree Dharma Sastha Temple A well known pilgrim centre of Kerala, Sabarimala lies in this district at a distance of 191 km. from Thiruvananthapuram and 210 km. away from Cochin. The holy shrine dedicated to Lord Ayyappa is situated 914 metres above sea level amidst dense forests in the rugged terrains of the Western Ghats. Lord Ayyappa is looked upon as the guardian of mountains and there are several shrines dedicated to him all along the Western Ghats. The festivals here are the Mandala Pooja, Makara Vilakku (December/January) and Vishu Kani (April). The temple is also open for pooja on the first 5 days of every Malayalam month. The vehicles go only up to Pampa and the temple, which is situated 5 km away from Pampa, can be reached only by trekking. During the festival period there are frequent buses to this place from Kochi, Thiruvananthapuram and Kottayam. 4 CONTENTS Pages 1. Foreword 7 2. Preface 9 3. Acknowledgements 11 4. History and scope of the District Census Handbook 13 5. Brief history of the district 15 6. Analytical Note 17 Village and Town Directory 105 Brief Note on Village and Town Directory 7. Section I - Village Directory (a) List of Villages merged in towns and outgrowths at 2011 Census (b) -

Accused Persons Arrested in Kannur District from 19.04.2020To25.04.2020
Accused Persons arrested in Kannur district from 19.04.2020to25.04.2020 Name of Name of Name of the Place at Date & Arresting the Court Name of the Age & Address of Cr. No & Police Sl. No. father of which Time of Officer, at which Accused Sex Accused Sec of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 560/2020 U/s 269,271,188 IPC & Sec ZHATTIYAL 118(e) of KP Balakrishnan NOTICE HOUSE, Chirakkal 25-04- Act &4(2)(f) VALAPATTA 19, Si of Police SERVED - J 1 Risan k RASAQUE CHIRAkkal Amsom 2020 at r/w Sec 5 of NAM Male Valapattanam F C M - II, amsom,kollarathin Puthiyatheru 12:45 Hrs Kerala (KANNUR) P S KANNUR gal Epidermis Decease Audinance 2020 267/2020 U/s KRISNA KRIPA NOTICE NEW MAHE 25-04- 270,188 IPC & RATHEESH J RAJATH NALAKATH 23, HOUSE,Nr. New Mahe SERVED - J 2 AMSOM MAHE 2020 at 118(e) of KP .S, SI OF VEERAMANI, VEERAMANI Male HEALTH CENTER, (KANNUR) F C M, PALAM 19:45 Hrs Act & 5 r/w of POLICE, PUNNOL THALASSERY KEDO 163/2020 U/s U/S 188, 269 Ipc, 118(e) of Kunnath house, kp act & sec 5 NOTICE 25-04- Abdhul 28, aAyyappankavu, r/w 4 of ARALAM SERVED - J 3 Abdulla k Aralam town 2020 at Sudheer k Rashhed Male Muzhakunnu kerala (KANNUR) F C M, 19:25 Hrs Amsom epidemic MATTANNUR diseases ordinance 2020 149/2020 U/s 188,269 NOTICE Pathiriyad 25-04- 19, Raji Nivas,Pinarayi IPC,118(e) of Pinarayi Vinod Kumar.P SERVED - A 4 Sajid.K Basheer amsom, 2020 at Male amsom Pinarayi KP Act & 4(2) (KANNUR) C ,SI of Police C J M, Mambaram 18:40 Hrs (f) r/w 5 of THALASSERY KEDO 2020 317/2020 U/s 188, 269 IPC & 118(e) of KP Act & Sec. -

212-223 HYDROLOGY of the KORAPUZHA ESTUARY, MALABAR, KERALA STATE* Central Marine Fisher
J. Mar. biol. Ass. India, 1959, 1 (2).' 212-223 HYDROLOGY OF THE KORAPUZHA ESTUARY, MALABAR, KERALA STATE* By S. V. SURYANARAYANA RAO & P. C. GEORGE Central Marine Fisheries Research Station, Mandapam Camp INTRODUCTION A preliminary enquiry on the hydrological and planktological conditions in the river mouth region was conducted during the period 1950-52 and the results pub lished in an abstract form (George, 1953a). This study was later extended to cover the 25 km seaward region of the estuary in 1954. As is well known, the Malabar coast is highly productive from the fisheries point of view and it was also observed that the sea waters of the inshore region off Calicut are rich in plank ton and nutrient salts (George, 19536). Results of the present investigation would therefore serve to assess the influence of land drainage in the enrichment and re plenishment of the coastal waters and allied factors. The west coast of India which is subjected to a heavy rainfall amounting to more than 300 cm per annum, is characterised by numerous short and swift rivers which carry the,enormous amounts of the waters from the Western Ghats into the sea. TOPOGRAPHY OF THE ESTUARY The Korapuzha is a short and shallow river, 52 km long and consists of the Elathur River which joins the Korapuzha backwater system close to the mouth of the estuary about a kilometer away from the sea and another stream running from the foot of the high mountain range surrounding the Kodiyanadumalai (700 Metres) which also empties into the back-waters near Kaniangode about 16 km away from the river mouth. -
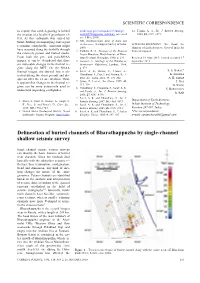
Delineation of Buried Channels of Bharathappuzha by Single-Channel Shallow Seismic Survey
SCIENTIFIC CORRESPONDENCE to explain that earth degassing is behind quake.usgs.gov/earthquakes/eventpage/ 12. Tronin, A. A., Int. J. Remote Sensing, the creation of a localized greenhouse ef- us20002926#general_summary (accessed 2000, 21, 3169–3177. fect. As this earthquake was caused by on 15 May 2015). thrust faulting accompanying convergent 3. GSI, Seismotectonic Atlas of India and tectonism, considerable emissions might its Environs, Geological Survey of India, ACKNOWLEDGEMENT. We thank the 2000. Ministry of Earth Sciences, Govt of India for have occurred along the foothills through 4. Valdiya, K. S., Geology of the Kumaon financial support. the relatively porous and faulted media. Lesser Himalaya, Wadia Institute of Hima- From both the pre- and post-NOAA layan Geology, Dehradun, 1980, p. 291. Received 16 May 2015; revised accepted 17 images, it can be deciphered that there 5. Gansser, A., Geology of the Himalayas, September 2015 are noticeable changes in the thermal re- Interscience Publishers, London, 1964, gime along the MFT. On the NOAA p. 273. thermal images, the thermal line is ob- 6. Saraf, A. K., Rawat, V., Tronin, A., S. S. BARAL* served during the stress periods and dis- Choudhury, S., Das, J. and Sharma, K., J. K. SHARMA appears after the release of stress. Thus, Geol. Soc. India, 2011, 77, 195–204. A. K. SARAF it appears that changes in the thermal re- 7. Qiang, Z. J. et al., Sci. China, 1999, 42, J. DAS 313–324. gime can be more extensively used to G. SINGH 8. Choudhury, S., Dasgupta, S., Saraf, A. K. S. BORGOHAIN understand impending earthquakes. -

Patterns of Discovery of Birds in Kerala Breeding of Black-Winged
Vol.14 (1-3) Jan-Dec. 2016 newsletter of malabar natural history society Akkulam Lake: Changes in the birdlife Breeding of in two decades Black-winged Patterns of Stilt Discovery of at Munderi Birds in Kerala Kadavu European Bee-eater Odonates from Thrissur of Kadavoor village District, Kerala Common Pochard Fulvous Whistling Duck A new duck species - An addition to the in Kerala Bird list of - Kerala for subscription scan this qr code Contents Vol.14 (1-3)Jan-Dec. 2016 Executive Committee Patterns of Discovery of Birds in Kerala ................................................... 6 President Mr. Sathyan Meppayur From the Field .......................................................................................................... 13 Secretary Akkulam Lake: Changes in the birdlife in two decades ..................... 14 Dr. Muhamed Jafer Palot A Checklist of Odonates of Kadavoor village, Vice President Mr. S. Arjun Ernakulam district, Kerala................................................................................ 21 Jt. Secretary Breeding of Black-winged Stilt At Munderi Kadavu, Mr. K.G. Bimalnath Kattampally Wetlands, Kannur ...................................................................... 23 Treasurer Common Pochard/ Aythya ferina Dr. Muhamed Rafeek A.P. M. A new duck species in Kerala .......................................................................... 25 Members Eurasian Coot / Fulica atra Dr.T.N. Vijayakumar affected by progressive greying ..................................................................... 27 -

A CONCISE REPORT on BIODIVERSITY LOSS DUE to 2018 FLOOD in KERALA (Impact Assessment Conducted by Kerala State Biodiversity Board)
1 A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) Editors Dr. S.C. Joshi IFS (Rtd.), Dr. V. Balakrishnan, Dr. N. Preetha Editorial Board Dr. K. Satheeshkumar Sri. K.V. Govindan Dr. K.T. Chandramohanan Dr. T.S. Swapna Sri. A.K. Dharni IFS © Kerala State Biodiversity Board 2020 All rights reserved. No part of this book may be reproduced, stored in a retrieval system, tramsmitted in any form or by any means graphics, electronic, mechanical or otherwise, without the prior writted permission of the publisher. Published By Member Secretary Kerala State Biodiversity Board ISBN: 978-81-934231-3-4 Design and Layout Dr. Baijulal B A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) EdItorS Dr. S.C. Joshi IFS (Rtd.) Dr. V. Balakrishnan Dr. N. Preetha Kerala State Biodiversity Board No.30 (3)/Press/CMO/2020. 06th January, 2020. MESSAGE The Kerala State Biodiversity Board in association with the Biodiversity Management Committees - which exist in all Panchayats, Municipalities and Corporations in the State - had conducted a rapid Impact Assessment of floods and landslides on the State’s biodiversity, following the natural disaster of 2018. This assessment has laid the foundation for a recovery and ecosystem based rejuvenation process at the local level. Subsequently, as a follow up, Universities and R&D institutions have conducted 28 studies on areas requiring attention, with an emphasis on riverine rejuvenation. I am happy to note that a compilation of the key outcomes are being published. -

Geostatistical Modelling of Sediment Chemistry of Ashtamudi Lake Using Gis and Study the Change During Past Several Years
Pramana Research Journal ISSN NO: 2249-2976 GEOSTATISTICAL MODELLING OF SEDIMENT CHEMISTRY OF ASHTAMUDI LAKE USING GIS AND STUDY THE CHANGE DURING PAST SEVERAL YEARS Grace K Mikhayel1, Prof .Chinnamma2 Malabar College of Engineering and Technology, Kerala Technology University, Thrissur (Dist),Kerala,India Abstract Water is valuable natural resources that essential to human survive and the ecosystems health. Water are comprises of coastal water bodies and fresh water bodies (lakes, river and groundwater). Since the past few decades, the increasing of anthropogenic activities especially in industrial area has effects to water bodies. This is the global issues which happening throughout the world and Kerala also face these problems. This study attempts to show the spatial distribution of sediment chemical parameters in the Ashtamudi Lake, Kollam and study the change during several past years. It also shows the partitioning of heavy metals in lake water. The Ashtamudi Lake is the second largest wetland ecosystem in Kerala. The lake is polluted by nearby factories, oil mills, boats, septic wastes etc. Sediment play an important role in elemental cycling in the aquatic environment and can be a sensitive indicator for monitoring contaminants in aquatic environment. GIS and remote sensing techniques can be used to make effective maps showing the effective spatial distribution of each parameters. Also sediment samples from various sample locations of Ashtamudi Lake will be collected and testing will be done accordingly. Index Term-Ashtamudi lake1, sediment sample2, sample point3, sample location4,parameters5 1. INTRODUCTION Water is valuable natural resources that essential to human survive and the ecosystems health. Water are comprises of coastal water bodies and fresh water bodies (lakes, river and groundwater).