Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Introduction WARNING! This Test System Is Capable of Producing Potentially Lethal Instrument Safety Voltages
Ultra System Overview Introduction Safety Introduction WARNING! This test system is capable of producing potentially lethal Instrument Safety voltages. All internal areas of the system must be accessed and Information serviced ONLY by trained and qualified personnel. Do not perform any servicing other than that contained in this documentation and This section describes the UltraFLEX read all safety information before attempting any operation or test system safety precautions. The maintenance procedures. test system generates and uses AC and DC high power and high voltage. The purpose of this section is to identify the hazards in the system to !!CAUTION! operation, service and applications personnel. Such personnel should Always wear safety glasses or safety goggles when working on the test system. read and understand this information before operating or servicing the system. !!CAUTION! Internal sheet metal portions of the test system may contain sharp edges, use care when working inside the test system. Page 1 of 3 Rev. 0736 Ultra System Overview Introduction General Safety Information Subsystem Safety Information The following general safety Table 1 identifies the safety concerns for the UltraFLEX subsystems. information is included in this section: Table 1 • General Safety Information • Safety Terms, Labels and Subsystem Safety Lockout/ Electrostatic Weight Liquid Safety Symbols Section Tagout Discharge Safety • Lockout/Tagout Computer Safety XX • ESD Damage Prevention and Control Measure Cooling XXX • Safety Certification DIB 24-Slot Interface XX • Material Handling Digital Signal Processor XX • Switches and Protective Barriers • Weight Safety Manipulator XX • Anti-Tip Fixture PDU XXX • Torque Specifications System Monitor and XXX Note Controller (SMC) Primary Support Board only. -

GUIDELINE Safety Data Sheets and Pathogen Safety Data Sheets
GUIDELINE UBC-RMS-OHS-GDL-14-001 Department of Risk Management Services Effective date: January 2, 2014 Review date: November 27, 2017 www.rms.ubc.ca Supersedes: Previous version Safety Data Sheets and Pathogen Safety Data Sheets 1. SCOPE This guideline provides directions for Regulatory and Best Practices for the use of Safety Data Sheets and Pathogen Safety Data Sheets (SDS and PSDS). It applies to all UBC employees working with chemicals and/or pathogens. 2. PURPOSE Safety Data Sheets (SDSs) and Pathogen Safety Data Sheets (PSDSs) are essential components in ensuring occupational health and safety within potentially hazardous environments. 3. BACKGROUND Under the new Workplace Hazardous Materials Information System (WHMIS 2015) the formerly known Material Safety Data Sheet (MSDS) became the Safety Data Sheet. The change is happening in several phases. After December 1, 2018, WHMIS 2015 will be the only system in use. Until then, employers are allowed in the workplace both MSDSs and SDSs. Important information regarding chemical, physiochemical, hazard/toxicity, required personal protective equipment, etc. are found on the SDS. In particular, WHMIS-related information will be found on the label of the agent of concern, with additional and more comprehensive information detailed in the SDS. Accordingly, the SDS for any new chemical, chemical mixture, or unknown agent should be fully reviewed prior to working with it. It is also important to note that suppliers are required to provide a SDS for each reagent (may be restricted to first time ordering). Similarly, Pathogen Safety Data Sheets (PSDSs) provide critical information regarding infectious materials, including the mode of transmission, disease symptoms, decontamination methods, PPE, and containment requirements. -

SAFETY DATA SHEET Page 1 of 2 SECTION 1: PRODUCT and COMPANY IDENTIFICATION Product: SURE-STEP™ Part Number: 3567000 Manufacturer: W
SAFETY DATA SHEET Page 1 of 2 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION Product: SURE-STEP™ Part Number: 3567000 Manufacturer: W. R. MEADOWS, INC. Address: 300 Industrial Drive Hampshire, Illinois 60140 Telephone: (847) 214-2100 In case of emergency, dial (800) 424-9300 (CHEMTREC) Revision Date: 6/8/2021 Product Use: Additive for Non-Slip Surfaces SECTION 2: HAZARDS IDENTIFICATION/EXPOSURE LIMITS HMIS HAZARD STATEMENTS |Health| | 1 | WARNING! |Flammability| | 1 | Direct contact may cause mechanical irritation. |Reactivity| | 0 | PRECAUTIONARY STATEMENTS |Personal Protection| | | Avoid direct contact. SECTION 3: HAZARDS COMPONENTS % by SARA Vapor Pressure LEL Chemical Name: CAS Number Weight 313 (mm Hg@20°C) (@25°C) 1. Polypropylene Homopolymer 9003-07-0 90-100 No N/A N/A N/A = Not Applicable Under the reporting requirements of Section 313 of Title III of the Superfund Amendments and Reauthorization Act of 1966 (SARA) and 40 CFR Part 372, chemicals listed on the 313 List (40 CFR Part 373.65) are identified under the heading "SARA 313." SECTION 4: EMERGENCY AND FIRST AID PROCEDURES EYE CONTACT: Flush eyes with water for fifteen minutes. If symptoms persist, seek medical attention. SKIN CONTACT: Rinse affected areas with water. INHALATION: Remove from exposure source. Treat symptomatically. INGESTION: Not expected to be an exposure route. Non-toxic if ingested. MOST IMPORTANT SYMPTOMS/EFFECTS, ACUTE AND CHRONIC: See Section Eleven for Symptoms/Effects. SECTION 5: FIRE AND EXPLOSIVES HAZARDS FLASHPOINT: Not applicable; product is a solid. EXTINGUISHING MEDIA: Carbon dioxide, dry chemical, or fine water spray. CHEMICAL/COMBUSTION HAZARDS: Melts in proximity to fires resulting in slippery surfaces. -

Safety Data Sheet Per GHS Standard Format
SDS Date: August, 2016 Safety Data Sheet Per GHS Standard Format SECTION 1: CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name: ABC® Asbestos Binding Compound No. 6421 White, No. 6422 Clear, No. 6423 Green Recommended Use of Product: Binder for Asbestos Fibers Information on the Supplier of the Safety Data Sheet Manufactured For: Emergency Telephone Numbers: Fiberlock Technologies, Inc. CHEM TEL: (U.S.): 1-800-255-3924 150 Dascomb Road (Outside the U.S.): 813-248-0585 Andover, MA 01810 P: 800-342-3755 F: 978-475-6205 SECTION 2: HAZARDS IDENTIFICATION Signal Word: WARNING GHS Label Statements Hazard Statements: Harmful if inhaled. Causes mild skin irritation. Causes serious eye irritation. GHS Classifications This product is considered hazardous by The 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute Toxicity-Inhalation (Vapors) Category 4 Acute Toxicity-Inhalation (Dust-mists) Category 2 Serious eye damage/eye irritation – Category 2 Skin sensitization – Category 1 Page 1 of 9 PRECAUTIONARY STATEMENTS Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Use personal protection (eye protection, gloves) during application. When grinding/sanding dry films, wear respiratory protection. Response: If on skin, wash with plenty of soap and water. If in eyes, rinse cautiously for several minutes. Remove contact lenses if present and easy to do. Continue rinsing. If inhaled, remove victim to fresh air. If exposed or concerned, get medical advice. Storage: Keep closures tight and containers upright to prevent leakage. KEEP FROM FREEZING. Product is non-combustible. Disposal: The coating and any contaminated diking material should be thoroughly air dried and collected into drums. -

Material Safety Data Sheet for Cell Cultures (Biosafety Level 1) 1
Material Safety Data Sheet: MSTO-211H / ID 300450 Material Safety Data Sheet for Cell Cultures (Biosafety Level 1) 1. Product Identification Name of cell line: MSTO-211H (CLS order no. 300450) Designation: Human Lung Mesothelioma cell line, permanent cell line 2. Company Identification CLS Cell Lines Service GmbH Dr. Eckener-Str. 8 D-69214 Eppelheim Germany Emergency phone number: +49 (0)6221 700799 3. Composition / Ingredients Unit: cryovial; frozen liquid Hazardous Ingredient(s) CAS no. Percentage EC no. Dimethyl Sulfoxide 67-68-5 10 200-664-3 Non-Hazardous Ingredient(s) Percentage DMEM, supplemented for freezing 60-80 FBS (Fetal Bovine Serum) 10-20 Cells 1 4. Hazards Identification Human source cell line Categorized as non-infectious and non-toxic 5. First Aid Measures Skin contact: Wash off immediately with plenty of water and soap. Eye contact: Flush eyes immediately with water for 10-15 minutes. Ingestion: If the material was swallowed, rinse the mouth with water. 6. Accidental Release Measures Use personal protective equipment Do not flush into surface water. Clean contaminated surface thoroughly. Autoclave before disposal into appropriated containers. CLS Cell Lines Service GmbH, Dr. Eckener-Straße 8, 69214 Eppelheim, Germany TEL: +49(0)6221 700799 FAX +49(0)6221 700717 [email protected] www.clsgmbh.de Page 1 of 2 Material Safety Data Sheet: MSTO-211H / ID 300450 7. Handling and storage Handling: Open only under a sterile workbench. Wear protective equipment. Handle as if containing infectious material. Storage: Keep the cryovial at -150°C (freezer) or at -196°C (liquid nitrogen vapour phase). 8. Personal Protection Hygienic measures Avoid the contact with skin, eyes and clothing. -
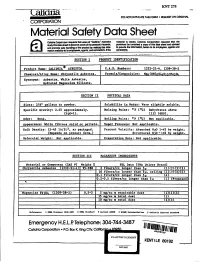
Material Safety Data Sheet
KNT275 00NOT DUPLICATE THIS FORM • REQUEST AN ORIGINAL CORPORATION Material Safety Data Sheet ClIfidria Corpor:lIiDn 18qU8&1I Ih8I users of "Calidrla" AsbeslDs maleri,aJ is resold, CaUdrla CorJIOraI!Dn requ8S11 lllal In. study this d8Ia shIIeI eo become aware of the procIucI's hazaJds. pu!dlIser be furnished a copy of this dala sheel and advised and pI'IlIIlllle safe handQng oj the pmducI by rnaJc!!911)iS 1nIor· . to prgvJde Iha ~. h8f8.in lD its emPJoyees. .,aand IIl8lIDn awDabIe to III ~. agents. and conIJ'8CIDI8.111he conlJ'8ClOfS. o .. - .~.• .:. .........._..,;.... SECTION I PRODUct IDENTIFICATION Product Name: CALIDR!A e ASBESTOS. C.A.S. Numbers: 1309-38-2 Chemical/AUo satHe Asbestos. Formula/Co osition: Synonyms: SECTION II PHYSICAL DATA Sizes: 3/8" ellets to owder. Solubilit in Water: Ve soluble. Specific Cravity: 2.45 approximately. Kelting Point: of (OC) Dehydrates above (H' 1). 1112 (600). Odor: None •. Point: OF (OC) Not a Heable. A earance: :Wbite fibrous solid or dIets. Not a Hcable. Bulk Density: 15-45 1b/ft3• as packaged. Percent Volatile: Absorbed 820 1-4% by weight. (De ends on roduct form.) Structural H?O - 13% b wei tht. Molecular Wei ht: Eva oration Rate: Nota Heable. SECTIOt~_UI HAZARDOUS INGREDIENTS 1(5) (5) tOsed (1309.-38~2) 0.5-2 .dust Ie'ereace., 1 OSHA SU""••d 1910.1001 Z IlSIIA St......... J Cunene ACCIA 6 MIOSJI "c_"dnlO1l S C.Udrtelace",a1 SUIld.... Emergency H.E.L.P. Telephone: 304-744-3487 Co6drfo Corpoiatlon • p.o. Box K. King CIty. CoOto PLAINTIFF'S EXHIBIT KENTflE 891$2 KFI-665 . -

Material Safety Data Sheet Revision Date 25-Feb-2011 Revision Number 1
Material Safety Data Sheet Revision Date 25-Feb-2011 Revision Number 1 1. PRODUCT AND COMPANY IDENTIFICATION Product Name MICROORGANISMS Synonyms No information available. Recommended Use Laboratory chemicals Company Emergency Telephone Number Remel INFOTRAC - 24 Hour Number: 1-800- 12076 Santa Fe Drive 535-5053 Lenexa, KS 66215 United States Outside of the United States, call 24 Telephone: 1-800-255-6730 Hour Number: 001-352-323-3500 (Call Fax:1-800-621-8251 Collect) 2. HAZARDS IDENTIFICATION CAUTION! Emergency Overview Potential Biohazard. Handle as potentially infectious. Appearance No information available. Physical State Solid odor No information available Target Organs No information available. Potential Health Effects Acute Effects Principle Routes of Exposure Eyes Avoid contact with eyes. Skin Avoid contact with skin. Inhalation May be harmful if inhaled. Ingestion Do not taste or swallow. Chronic Effects None known See Section 11 for additional Toxicological information. Aggravated Medical Conditions No information available. 3. COMPOSITION/INFORMATION ON INGREDIENTS Haz/Non-haz Component CAS-No Weight % Microorganisms RR-47883-1 100 _____________________________________________________________________________________________ Page 1 / 7 Thermo Fisher Scientific - MICROORGANISMS Revision Date 25-Feb-2011 _____________________________________________________________________________________________ 4. FIRST AID MEASURES Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Call a physician immediately. Skin Contact Wash off immediately with soap and plenty of water. Inhalation Move to fresh air. Obtain medical attention. Ingestion Rinse mouth. Call a physician or Poison Control Center immediately. Notes to Physician Treat symptomatically. 5. FIRE-FIGHTING MEASURES Flash Point No information available. Method No information available. Autoignition Temperature No information available. -

Safety Data Sheet
SAFETY DATA SHEET This safety data sheet was created pursuant to the requirements of: US OSHA Hazard Communication Standard (29 CFR 1910.1200) and Canada WHMIS 2015 which includes the amended Hazardous Products Act (HPA) and the Hazardous Products Regulation (HPR) Issuing Date 17-Apr-2020 Revision Date 17-Apr-2020 Revision Number 1 1. Identification Product identifier Product Name MetalWorksÔ Blades Classics by Armstrong Other means of identification Synonyms Linear Blades Recommended use of the chemical and restrictions on use Recommended use Ceilings Restrictions on use No information available. Details of the supplier of the safety data sheet Initial supplier identifier Supplier Address Armstrong World Industries Armstrong World Industries 255 Montpellier Blvd 2500 Columbia Avenue St. Laurent, Quebec Lancaster, PA 17603 Canada H4N 2G3 Tel: 877-276-7876 Tel: 877-276-7876 [email protected] [email protected] Emergency telephone number Emergency Telephone 1-800-255-3924 (ChemTel) 2. Hazard(s) identification Classification This product is an article as defined by the OSHA Hazard Communication Standard 2012 (29 CFR 1910.1200) and Canada WHMIS 2015, which includes the amended Hazardous Products Act (HPA). No exposure to hazardous chemicals is expected to occur during intended product use. Misuse of the product may result in exposure to hazards. Label elements Hazard statements Not classified. Other information No information available. _____________________________________________________________________________________________ -

Gasoline (All Grades)
SAFETY DATA SHEET Gasoline (All Grades) Section 1. Identification Product name : Gasoline (All Grades) Product code : Not available. Synonyms : Gasoline, Unleaded Gasoline, Regular Gasoline, Premium Gasoline, Oxyfuel, Reformulated Gasoline Relevant identified uses of the substance or mixture and uses advised against Product use : Use in fuel - Industrial use Area of application : Industrial applications. Manufacturer : HollyFrontier Refining & Marketing LLC 2828 North Harwood Suite 1300 Dallas, Texas 75201 USA Customer Service: (888) 286-8836 Emergency telephone : CHEMTREC® (800) 424-9300 number CCN 201319 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : H224 FLAMMABLE LIQUIDS - Category 1 substance or mixture H315 SKIN IRRITATION - Category 2 H319 EYE IRRITATION - Category 2A H340 GERM CELL MUTAGENICITY - Category 1 H350 CARCINOGENICITY - Category 1B H361 TOXIC TO REPRODUCTION (Fertility) - Category 2 H361 TOXIC TO REPRODUCTION (Unborn child) - Category 2 H336 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 H373 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) (liver) - Category 2 H304 ASPIRATION HAZARD - Category 1 Percentage of the mixture consisting of ingredient(s) of unknown dermal toxicity: 9% Percentage of the mixture consisting of ingredient(s) of unknown inhalation toxicity: 4% GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : H224 - Extremely flammable liquid and vapor. H319 - Causes serious eye irritation. H315 - Causes skin irritation. H340 - May cause genetic defects. H350 - May cause cancer. H361 - Suspected of damaging fertility or the unborn child. H304 - May be fatal if swallowed and enters airways. H336 - May cause drowsiness or dizziness. H373 - May cause damage to organs through prolonged or repeated exposure. -

Safety Data Sheet (Sds)
SAFETY DATA SHEET (SDS) A GUIDE TO THE F O R M A T A N D CONTENTS OF THE SDS FOR AUTO SHOP WORKERS SAFE SHOPS PROGRAM BOSTON PUBLIC HEALTH COMMISSION WHAT ARE SAFETY DATA SHEETS (SDS)? • They provide information about the chemicals in the products you work with • Properties of each chemical • The physical, health, and environmental hazards • How quickly the chemical may cause harm (acute, chronic) • Where on the body the chemical may cause harm (local, systemic, target organ, etc.) • Protective measures • Safety precautions for handling, storing, and transporting the chemical • Chemical manufacturers and importers are required to: • Evaluate the hazards of the chemicals they produce or import • Prepare labels and SDS to explain the hazard information and make them available to users • Employers must have them available to workers. Employers may: • Keep the SDSs in a binder in the work area • Keep the SDSs on computers, as long as the employees have immediate access to the information without leaving their work area when needed, and as long as a back-up is available for rapid access to the SDS in the case of a power outage or other emergency • Designate a person(s) responsible for obtaining and maintaining the SDSs • If the employer does not have an SDS, the employer or designated person(s) should contact the manufacturer to obtain one 2 HEALTH EFFECTS: TERMS • Acute effect: Symptoms from exposure show up quickly • Eye contact: May cause severe irritation and permanent damage • Skin contact: May cause reddening, blistering Blistering or -

SAFETY DATA SHEET SDS No
SAFETY DATA SHEET SDS No. 466A Revision Date: 8/23/2019 Version: 5.0 GHS Compliant Section 1 - Identification of the substance/mixture and of the company/undertaking 1.1 Product Identifier: Part A for: Task® 6; Part B for: TerraCon® 55 and Urethane 4040 1.2 General Use: Polyurethane Elastomer 1.3 Manufacturer: Smooth-On, Inc., 5600 Lower Macungie Rd., Macungie, PA 18062 Phone (610) 252-5800, [email protected] 1.4 Emergency Contact: Chem-Tel Domestic: 800-255-3924 International: 813-248-0585 Section 2 – Hazard(s) Identification 2.1 Classification of the substance or mixture: H315 Skin corrosion/irritation – Category 2 H317 Skin sensitization – Category 1 H320 Eye Damage/irritation - Category 2B H332 Acute toxicity, inhalation – Category 4 H334 Respiratory Sensitization – Category 1 H335 Specific target organ toxicity – single exposure – Category 3 (respiratory) H351 Carcinogenicity – Category 2 H360 Reproductive - Category 1 H373 Specific Target Organ Toxicity, repeated exposure Category 2 (respiratory) 2.2 GHS Label elements, including precautionary statements Pictogram(s): Signal word: Danger Health Hazards: H315 Causes skin irritation H317 May cause an allergic skin reaction H320 Causes eye irritation H332 Harmful if inhaled H334 May cause allergy or asthma symptoms or breathing difficulties if inhaled H335 May cause respiratory irritation H351 Suspected of causing cancer H360 May damage fertility or the unborn child H373 May cause damage to organs through prolonged or repeated exposure General Precautions: P101 If medical advice is needed, have product container or label at hand. P102 Keep out of reach of children. P103 Read label before use. Prevention Precautions: P201 Obtain special instructions before use. -

[email protected] +1-703-527-3887 (International) Website
Date of Issue: 06 September 2016 SAFETY DATA SHEET 1. SUBSTANCE AND SOURCE IDENTIFICATION Product Identifier SRM Number: 1649b SRM Name: Urban Dust Other Means of Identification: Not applicable. Recommended Use of This Material and Restrictions of Use This Standard Reference Material (SRM) is intended for use in evaluating analytical methods for the determination of selected polycyclic aromatic hydrocarbons (PAHs), nitro-substituted PAHs (nitro-PAHs), polychlorinated biphenyl (PCB) congeners, chlorinated pesticides, and inorganic constituents in atmospheric particulate material and similar matrices. A unit of SRM 1649b consists of a bottle containing 2 g of particulate material. Company Information National Institute of Standards and Technology Standard Reference Materials Program 100 Bureau Drive, Stop 2300 Gaithersburg, Maryland 20899-2300 Telephone: 301-975-2200 Emergency Telephone ChemTrec: FAX: 301-948-3730 1-800-424-9300 (North America) E-mail: [email protected] +1-703-527-3887 (International) Website: http://www.nist.gov/srm 2. HAZARDS IDENTIFICATION Note: The concentration of PAHs, nitro-PAHs, PCB congeners, chlorinated pesticides, and inorganic constituents in SRM 1649b is below the cut-off value/concentration limit for SDS information as required by OSHA 29 CFR 1910.1200. The health and physical hazard information provided in this SDS is for urban dust as a particulate matter. Classification Physical Hazard: Not classified. Health Hazard: Not classified. Label Elements Symbol: No Symbol Signal Word: No Signal Word Hazard Statement(s): Not applicable. Precautionary Statement(s): Not applicable. Hazards Not Otherwise Classified: Not applicable. Ingredients(s) with Unknown Acute Toxicity: Not applicable. 3. COMPOSITION AND INFORMATION ON HAZARDOUS INGREDIENTS Substance: Urban Dust Other Designations: Dust; atmospheric particulate matter; urban particulate matter Components are listed in compliance with OSHA’s 29 CFR 1910.1200; for the actual values see the Certificate of Analysis.