Cnidarian and Ctenophore Lab Manual MBL Embryology Course 2010
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Sexual Reproduction 7.2: Meiosis 7.3: Errors in Meiosis
Concepts of Biology Chapter 6 | Reproduction at the Cellular Level 135 6 | REPRODUCTION AT THE CELLULAR LEVEL Figure 6.1 A sea urchin begins life as a single cell that (a) divides to form two cells, visible by scanning electron microscopy. After four rounds of cell division, (b) there are 16 cells, as seen in this SEM image. After many rounds of cell division, the individual develops into a complex, multicellular organism, as seen in this (c) mature sea urchin. (credit a: modification of work by Evelyn Spiegel, Louisa Howard; credit b: modification of work by Evelyn Spiegel, Louisa Howard; credit c: modification of work by Marco Busdraghi; scale-bar data from Matt Russell) Chapter Outline 6.1: The Genome 6.2: The Cell Cycle 6.3: Cancer and the Cell Cycle 6.4: Prokaryotic Cell Division Introduction The individual sexually reproducing organism—including humans—begins life as a fertilized egg, or zygote. Trillions of cell divisions subsequently occur in a controlled manner to produce a complex, multicellular human. In other words, that original single cell was the ancestor of every other cell in the body. Once a human individual is fully grown, cell reproduction is still necessary to repair or regenerate tissues. For example, new blood and skin cells are constantly being produced. All multicellular organisms use cell division for growth, and in most cases, the maintenance and repair of cells and tissues. Single-celled organisms use cell division as their method of reproduction. 6.1 | The Genome By the end of this section, you will be able to: • Describe the prokaryotic and eukaryotic genome • Distinguish between chromosomes, genes, and traits The continuity of life from one cell to another has its foundation in the reproduction of cells by way of the cell cycle. -

Microbiota Differences of the Comb Jelly Mnemiopsis Leidyi in Native and Invasive Sub-Populations
bioRxiv preprint doi: https://doi.org/10.1101/601419; this version posted April 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Microbiota differences of the comb jelly Mnemiopsis leidyi in native and invasive sub‐populations Cornelia Jaspers1*, Nancy Weiland‐Bräuer3, Martin Fischer3, Sven Künzel4, Ruth A. Schmitz3 and Thorsten B.H. Reusch1 1GEOMAR Helmholtz Centre for Ocean Research Kiel, Marine Evolutionary Ecology, Kiel, Germany 2Institute for General Microbiology, Christian‐Albrechts‐Universität zu Kiel, Kiel, Germany 3Max Planck Institute for Evolutionary Biology, Plön, Germany *corresponding author: [email protected], +49‐431‐600‐4560, ORCID: 0000‐0003‐2850‐4131 Keywords: Comb jelly, metaorganism, bacteria, species translocations, invasive species 1 bioRxiv preprint doi: https://doi.org/10.1101/601419; this version posted April 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT The translocation of non‐indigenous species around the world, especially in marine systems, is a matter of concern for biodiversity conservation and ecosystem functioning. While specific traits are often recognized to influence establishment success of non‐indigenous species, the impact of the associated microbial community for the fitness, performance and invasion success of basal marine metazoans remains vastly unknown. In this study we compared the microbiota community composition of the invasive ctenophore Mnemiopsis leidyi in different native and invasive sub‐populations along with characterization of the genetic structure of the host. By 16S rRNA gene amplicon sequencing we showed that the sister group to all metazoans, namely ctenophores, harbored a distinct microbiota on the animal host, which significantly differed across two major tissues, namely epidermis and gastrodermis. -

The Evolution of Siphonophore Tentilla for Specialized Prey Capture in the Open Ocean
The evolution of siphonophore tentilla for specialized prey capture in the open ocean Alejandro Damian-Serranoa,1, Steven H. D. Haddockb,c, and Casey W. Dunna aDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520; bResearch Division, Monterey Bay Aquarium Research Institute, Moss Landing, CA 95039; and cEcology and Evolutionary Biology, University of California, Santa Cruz, CA 95064 Edited by Jeremy B. C. Jackson, American Museum of Natural History, New York, NY, and approved December 11, 2020 (received for review April 7, 2020) Predator specialization has often been considered an evolutionary makes them an ideal system to study the relationships between “dead end” due to the constraints associated with the evolution of functional traits and prey specialization. Like a head of coral, a si- morphological and functional optimizations throughout the organ- phonophore is a colony bearing many feeding polyps (Fig. 1). Each ism. However, in some predators, these changes are localized in sep- feeding polyp has a single tentacle, which branches into a series of arate structures dedicated to prey capture. One of the most extreme tentilla. Like other cnidarians, siphonophores capture prey with cases of this modularity can be observed in siphonophores, a clade of nematocysts, harpoon-like stinging capsules borne within special- pelagic colonial cnidarians that use tentilla (tentacle side branches ized cells known as cnidocytes. Unlike the prey-capture apparatus of armed with nematocysts) exclusively for prey capture. Here we study most other cnidarians, siphonophore tentacles carry their cnidocytes how siphonophore specialists and generalists evolve, and what mor- in extremely complex and organized batteries (3), which are located phological changes are associated with these transitions. -

Invasive Species: a Challenge to the Environment, Economy, and Society
Invasive Species: A challenge to the environment, economy, and society 2016 Manitoba Envirothon 2016 MANITOBA ENVIROTHON STUDY GUIDE 2 Acknowledgments The primary author, Manitoba Forestry Association, and Manitoba Envirothon would like to thank all the contributors and editors to the 2016 theme document. Specifically, I would like to thank Robert Gigliotti for all his feedback, editing, and endless support. Thanks to the theme test writing subcommittee, Kyla Maslaniec, Lee Hrenchuk, Amie Peterson, Jennifer Bryson, and Lindsey Andronak, for all their case studies, feedback, editing, and advice. I would like to thank Jacqueline Montieth for her assistance with theme learning objectives and comments on the document. I would like to thank the Ontario Envirothon team (S. Dabrowski, R. Van Zeumeren, J. McFarlane, and J. Shaddock) for the preparation of their document, as it provided a great launch point for the Manitoba and resources on invasive species management. Finally, I would like to thank Barbara Fuller, for all her organization, advice, editing, contributions, and assistance in the preparation of this document. Olwyn Friesen, BSc (hons), MSc PhD Student, University of Otago January 2016 2016 MANITOBA ENVIROTHON STUDY GUIDE 3 Forward to Advisors The 2016 North American Envirothon theme is Invasive Species: A challenge to the environment, economy, and society. Using the key objectives and theme statement provided by the North American Envirothon and the Ontario Envirothon, the Manitoba Envirothon (a core program of Think Trees – Manitoba Forestry Association) developed a set of learning outcomes in the Manitoba context for the theme. This document provides Manitoba Envirothon participants with information on the 2016 theme. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1999 Academic Press. This manuscript is an author version with the final publication available and may be cited as: Young, C. M. (1999). Marine invertebrate larvae. In E. Knobil & J. D. Neill (eds.), Encyclopedia of Reproduction, 3. (pp. 89-97). London, England, and San Diego, CA: Academic Press. --------1111------- Marine Invertebrate Larvae Craig M. Young Harbor Branch Oceanographic Institution 1. What Is a Larva? metamorphOSiS Morphological and physiological changes II. The Production of Larvae that occur during the transition from the larval phase to iII. Larval forms and Diversity the juvenile phase: often coincides with settlement in ben IV. Larval Feeding and Nutrition thic species. V. Larval Orientation, Locomotion, Dispersal, and mixed development A developmental mode that includes a Mortality brooded or encapsulated embryonic stage as well as a free VI. Larval Settlement and Metamorphosis swimming larval stage. VlI. Ecological and Evolutionary Significance of Larvae planktotrophic larva A feeding larva that obtains at least part VlIl. Economic and Medical Importance of Larvae of its nutritional needs from either particulate or dissolved exogenous sources. Planktotrophic larvae generally hatch from small, transparent eggs. GLOSSARY settlement The permanent transition of a larva from the plankton to the benthos. In sessile organisms, settlement atrochal larva A uniformly ciliated larva (cilia not arranged is marked by adhesion to the substratum. It is often closely in distinct bands). associated with metamorphosis and may involve habitat se competent larva A larva that is physiologically and morpho lection. -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis ✉ Aissam Ikmi 1,2 , Petrus J
ARTICLE https://doi.org/10.1038/s41467-020-18133-0 OPEN Feeding-dependent tentacle development in the sea anemone Nematostella vectensis ✉ Aissam Ikmi 1,2 , Petrus J. Steenbergen1, Marie Anzo 1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2 & Matthew C. Gibson2,4 In cnidarians, axial patterning is not restricted to embryogenesis but continues throughout a prolonged life history filled with unpredictable environmental changes. How this develop- 1234567890():,; mental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. Here we utilize the tentacles of the sea anemone Nematostella vectensis as an experimental paradigm for developmental patterning across distinct life history stages. By analyzing over 1000 growing polyps, we find that tentacle progression is stereotyped and occurs in a feeding-dependent manner. Using a combination of genetic, cellular and molecular approaches, we demonstrate that the crosstalk between Target of Rapamycin (TOR) and Fibroblast growth factor receptor b (Fgfrb) signaling in ring muscles defines tentacle primordia in fed polyps. Interestingly, Fgfrb-dependent polarized growth is observed in polyp but not embryonic tentacle primordia. These findings show an unexpected plasticity of tentacle development, and link post-embryonic body patterning with food availability. 1 Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2 Stowers Institute for Medical Research, Kansas City, MO 64110, -

Crustacea: Amphipoda: Hyperiidea: Hyperiidae), with the Description of a New Genus to Accommodate H
Zootaxa 3905 (2): 151–192 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3905.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:A47AE95B-99CA-42F0-979F-1CAAD1C3B191 A review of the hyperiidean amphipod genus Hyperoche Bovallius, 1887 (Crustacea: Amphipoda: Hyperiidea: Hyperiidae), with the description of a new genus to accommodate H. shihi Gasca, 2005 WOLFGANG ZEIDLER South Australian Museum, North Terrace, Adelaide, South Australia 5000, Australia. E-mail [email protected] Table of contents Abstract . 151 Introduction . 152 Material and methods . 152 Systematics . 153 Suborder Hyperiidea Milne-Edwards, 1830 . 153 Family Hyperiidae Dana, 1852 . 153 Genus Hyperoche Bovallius, 1887 . 153 Key to the species of Hyperoche Bovallius, 1887 . 154 Hyperoche medusarum (Kröyer, 1838) . 155 Hyperoche martinezii (Müller, 1864) . 161 Hyperoche picta Bovallius, 1889 . 165 Hyperoche luetkenides Walker, 1906 . 168 Hyperoche mediterranea Senna, 1908 . 173 Hyperoche capucinus Barnard, 1930 . 177 Hyperoche macrocephalus sp. nov. 180 Genus Prohyperia gen. nov. 182 Prohyperia shihi (Gasca, 2005) . 183 Acknowledgements . 186 References . 186 Abstract This is the first comprehensive review of the genus Hyperoche since that of Bovallius (1889). This study is based primarily on the extensive collections of the ZMUC but also on more recent collections in other institutions. Seven valid species are recognised in this review, including one described as new to science. Two new characters were discovered; the first two pereonites are partially or wholly fused dorsally and the coxa of pereopod 7 is fused with the pereonite. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Evolution of the TGF-B Signaling Pathway and Its Potential Role in the Ctenophore, Mnemiopsis Leidyi
Evolution of the TGF-b Signaling Pathway and Its Potential Role in the Ctenophore, Mnemiopsis leidyi Kevin Pang1, Joseph F. Ryan2, Andreas D. Baxevanis2, Mark Q. Martindale1* 1 Kewalo Marine Laboratory, Pacific Biosciences Research Center, University of Hawaii at Manoa, Honolulu, Hawaii, United States of America, 2 Genome Technology Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland, United States of America Abstract The TGF-b signaling pathway is a metazoan-specific intercellular signaling pathway known to be important in many developmental and cellular processes in a wide variety of animals. We investigated the complexity and possible functions of this pathway in a member of one of the earliest branching metazoan phyla, the ctenophore Mnemiopsis leidyi. A search of the recently sequenced Mnemiopsis genome revealed an inventory of genes encoding ligands and the rest of the components of the TGF-b superfamily signaling pathway. The Mnemiopsis genome contains nine TGF-b ligands, two TGF-b- like family members, two BMP-like family members, and five gene products that were unable to be classified with certainty. We also identified four TGF-b receptors: three Type I and a single Type II receptor. There are five genes encoding Smad proteins (Smad2, Smad4, Smad6, and two Smad1s). While we have identified many of the other components of this pathway, including Tolloid, SMURF, and Nomo, notably absent are SARA and all of the known antagonists belonging to the Chordin, Follistatin, Noggin, and CAN families. This pathway likely evolved early in metazoan evolution as nearly all components of this pathway have yet to be identified in any non-metazoan. -

Ctenophore Immune Cells Produce Chromatin Traps in Response to Pathogens and NADPH- Independent Stimulus
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.09.141010; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Ctenophore immune cells produce chromatin traps in response to pathogens and NADPH- independent stimulus Authors and Affiliations: Lauren E. Vandepasa,b,c*†, Caroline Stefanic†, Nikki Traylor-Knowlesd, Frederick W. Goetzb, William E. Brownee, Adam Lacy-Hulbertc aNRC Research Associateship Program; bNorthwest Fisheries Science Center, National Oceanographic and Atmospheric Administration, Seattle, WA 98112; cBenaroya Research Institute at Virginia Mason, Seattle, WA 98101; dUniversity of Miami Rosenstiel School of Marine and Atmospheric Sciences, Miami, FL 33149; eUniversity of Miami Department of Biology, Coral Gables, FL 33146; *Corresponding author; †Authors contributed equally Key Words: Ctenophore; ETosis; immune cell evolution Abstract The formation of extracellular DNA traps (ETosis) is a mechanism of first response by specific immune cells following pathogen encounters. Historically a defining behavior of vertebrate neutrophils, cells capable of ETosis were recently discovered in several invertebrate taxa. Using pathogen and drug stimuli, we report that ctenophores – thought to represent the earliest- diverging animal lineage – possess cell types capable of ETosis, suggesting that this cellular immune response behavior likely evolved early in the metazoan stem lineage. Introduction Immune cells deploy diverse behaviors during pathogen elimination, including phagocytosis, secretion of inflammatory cytokines, and expulsion of nuclear material by casting extracellular DNA “traps” termed ETosis. Specific immune cell types have not been identified in early diverging non-bilaterian phyla and thus conservation of cellular immune behaviors across Metazoa remains unclear. -

Hazardous Marine Life
JOIN US AT DAN.ORG HAZARDOUS MARINE LIFE STINGS Cnidarians (nematocyst-carrying species) are HEALTH & DIVING REFERENCE SERIES responsible for more evenomations than any other marine phylum. These organisms contain stinging cells called cnidocyte that excel at venom delivery. 6 West Colony Place Durham, NC 27705 USA HAZARDOUS JELLYFISH PHONE: +1-919-684-2948 Of all the cnidarians, jellyfish cause the most MARINE LIFE: DAN EMERGENCY HOTLINE: +1-919-684-9111 frequent and severe human injuries. These result LEARN MORE AT DAN.ORG/HEALTH STINGS from direct contact with tentacles, and though painful, are not typically life threatening. PORTUGUESE MAN-OF-WAR Portuguese man-of-wars are free-floating cnidarians characterized by blue gas-filled bladders and long tentacles that drift on the ocean’s surface. Contact with a man-of-war’s Part #: 013-1040 Rev. 7.27.15 tentacles can cause significant pain and systemic symptoms. Their tentacles contain cnidocytes that deliver a potent proteic neurotoxin. Despite its resemblance to jellyfish, Portuguese man-of-war are more closely related to fire coral than to true jellyfish. HYDROIDS Although hydroids look like plants, these feathery cnidarians are actually a colony of small zooids that work together as a functioning animal. Like all cnidarians, these animals are armed with stinging cnidocytes. Hydroids, Portuguese man-of-war and fire coral belong to the same family, and contact with them can cause very similar reactions. Granted, their smaller contact area usually results in more localized and less dramatic reactions. FIRE CORAL Fire coral are colonial marine cnidarians that cause a mild to moderate burning reaction when touched. -
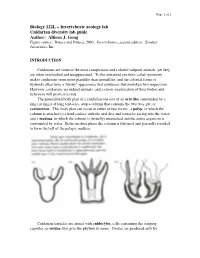
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators.