Lab 2: Microfossils
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Can You Engineer an Insect Exoskeleton?
Page 1 of 14 Can you Engineer an Insect Exoskeleton? Submitted by Catherine Dana and Christina Silliman EnLiST Entomology Curriculum Developers Department of Entomology, University of Illinois Grade level targeted: 4th Grade, but can be easily adapted for later grades Big ideas: Insect exoskeleton, engineering, and biomimicry Main objective: Students will be able to design a functional model of an insect exoskeleton which meets specific physical requirements based on exoskeleton biomechanics Lesson Summary We humans have skin and bones to protect us and to help us stand upright. Insects don’t have bones or skin but they are protected from germs, physical harm, and can hold their body up on their six legs. This is all because of their hard outer shell, also known as their exoskeleton. This hard layer does more than just protect insects from being squished, and students will get to explore some of the many ways the exoskeleton protects insects by building one themselves! For this fourth grade lesson, students will work together in teams to use what they learn about exoskeleton biomechanics to design and build a protective casing. To complete the engineering design cycle, students can use what they learn from testing their case to redesign and re-build their prototype. Prerequisites No prior knowledge is required for this lesson. Students should be introduced to the general form of an insect to facilitate identification of the exoskeleton. Live insects would work best for this, but pictures and diagrams will work as well. Instruction Time 45 – 60 minutes Next Generation Science Standards (NGSS) Framework Alignment Disciplinary Core Ideas LS1.A: Structure and Function o Plants and animals have both internal and external structures that serve various functions in growth, survival, behavior, and reproduction. -

Do You Think Animals Have Skeletons Like Ours?
Animal Skeletons Do you think animals have skeletons like ours? Are there any bones which might be similar? Vertebrate or Invertebrate § Look at the words above… § What do you think the difference is? § Hint: Break the words up (Vertebrae) Vertebrates and Invertebrates The difference between vertebrates and invertebrates is simple! Vertebrates have a backbone (spine)… …and invertebrates don’t Backbone (spine) vertebrate invertebrate So, if the animal has a backbone or a ‘vertebral column’ it is a ‘Vertebrate’ and if it doesn’t, it is called an ‘Invertebrate.’ It’s Quiz Time!! Put this PowerPoint onto full slideshow before starting. You will be shown a series of animals, click if you think it is a ‘Vertebrate’ or an ‘Invertebrate.’ Dog VertebrateVertebrate or InvertebrateInvertebrate Worm VertebrateVertebrate or InvertebrateInvertebrate Dinosaur VertebrateVertebrate or InvertebrateInvertebrate Human VertebrateVertebrate or InvertebrateInvertebrate Fish VertebrateVertebrate or InvertebrateInvertebrate Jellyfish VertebrateVertebrate or InvertebrateInvertebrate Butterfly VertebrateVertebrate or InvertebrateInvertebrate Types of Skeleton § Now we know the difference between ‘Vertebrate’ and ‘Invertebrate.’ § Let’s dive a little deeper… A further classification of skeletons comes from if an animal has a skeleton and where it is. All vertebrates have an endoskeleton. However invertebrates can be divided again between those with an exoskeleton and those with a hydrostatic skeleton. vertebrate invertebrate endoskeleton exoskeleton hydrostatic skeleton What do you think the words endoskeleton, exoskeleton and hydrostatic skeleton mean? Endoskeletons Animals with endoskeletons have Endoskeletons are lighter skeletons on the inside than exoskeletons. of their bodies. As the animal grows so does their skeleton. Exoskeletons Animals with exoskeletons Watch the following have clip to see how they shed their skeletons on their skeletons the outside! (clip the crab below). -

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
bioRxiv preprint doi: https://doi.org/10.1101/587352; this version posted May 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bjorbækmo et al., 23.03.2019 – preprint copy - BioRxiv The planktonic protist interactome: where do we stand after a century of research? Marit F. Markussen Bjorbækmo1*, Andreas Evenstad1* and Line Lieblein Røsæg1*, Anders K. Krabberød1**, and Ramiro Logares2,1** 1 University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N- 0316 Oslo, Norway 2 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, ES-08003, Barcelona, Catalonia, Spain * The three authors contributed equally ** Corresponding authors: Ramiro Logares: Institute of Marine Sciences (ICM-CSIC), Passeig Marítim de la Barceloneta 37-49, 08003, Barcelona, Catalonia, Spain. Phone: 34-93-2309500; Fax: 34-93-2309555. [email protected] Anders K. Krabberød: University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N-0316 Oslo, Norway. Phone +47 22845986, Fax: +47 22854726. [email protected] Abstract Microbial interactions are crucial for Earth ecosystem function, yet our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2,500 ecological interactions from ~500 publications, spanning the last 150 years. -

SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
SHELLS IN ACID Adapted from NAMEPA’s An Educator’s Guide to the Marine Environment: Shells in Acid PURPOSE Students will test the strength of normal seashells versus shells that have been soaked in vinegar to simulate the weakening effect of ocean acidification. Students identify the correlation between decreasing oceanic pH (ocean acidification) and the weakening of shells and discuss the effect this could have on the health of shellfish in the world’s oceans. MATERIALS (PER GROUP OF 4) • *white vinegar • *small, thin seashells • *non-reactive containers (glass beakers, Pyrex, measuring glass) • *water • heavy books (several) • paper towels For #6: • shells (1 per student) • snack size plastic bags (1 per student) • Small amount of vinegar • magnifying glass *Before beginning this activity, shells should be pre-soaked overnight in a 1:1 solution of vinegar and fresh water. PROCEDURE 1. Engage/Elicit Ask the students to give examples of different species of shellfish. Answers may include clams, oysters, mussels, scallops, etc. Ask students why and where they have seen these creatures. Students’ knowledge may come from eating seafood, or perhaps from having seen them in an aquarium, a marina or in coastal areas. Ask the students why these animals are important to the marine environment and to human beings. 2. Explore Lay out an assemblage of the non-soaked shells. Have the students observe the shells. Allow the students to handle the shells and ask them why the development of shells is advantageous to such animals. Explain that shellfish are invertebrates, meaning that instead of having an internal skeleton like humans, invertebrates produce a hard, protective covering. -

Outer Continental Shelf Environmental Assessment Program, Final Reports of Principal Investigators. Volume 71
Outer Continental Shelf Environmental Assessment Program Final Reports of Principal Investigators Volume 71 November 1990 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Ocean Service Office of Oceanography and Marine Assessment Ocean Assessments Division Alaska Office U.S. DEPARTMENT OF THE INTERIOR Minerals Management Service Alaska OCS Region OCS Study, MMS 90-0094 "Outer Continental Shelf Environmental Assessment Program Final Reports of Principal Investigators" ("OCSEAP Final Reports") continues the series entitled "Environmental Assessment of the Alaskan Continental Shelf Final Reports of Principal Investigators." It is suggested that reports in this volume be cited as follows: Horner, R. A. 1981. Bering Sea phytoplankton studies. U.S. Dep. Commer., NOAA, OCSEAP Final Rep. 71: 1-149. McGurk, M., D. Warburton, T. Parker, and M. Litke. 1990. Early life history of Pacific herring: 1989 Prince William Sound herring egg incubation experiment. U.S. Dep. Commer., NOAA, OCSEAP Final Rep. 71: 151-237. McGurk, M., D. Warburton, and V. Komori. 1990. Early life history of Pacific herring: 1989 Prince William Sound herring larvae survey. U.S. Dep. Commer., NOAA, OCSEAP Final Rep. 71: 239-347. Thorsteinson, L. K., L. E. Jarvela, and D. A. Hale. 1990. Arctic fish habitat use investi- gations: nearshore studies in the Alaskan Beaufort Sea, summer 1988. U.S. Dep. Commer., NOAA, OCSEAP Final Rep. 71: 349-485. OCSEAP Final Reports are published by the U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Ocean Service, Ocean Assessments Division, Alaska Office, Anchorage, and primarily funded by the Minerals Management Service, U.S. Department of the Interior, through interagency agreement. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Evaluating the Biological Pump Efficiency of the Last Glacial Maximum Ocean Using Δ13c Anne L
Evaluating the Biological Pump Efficiency of the Last Glacial Maximum Ocean using δ13C Anne L. Morée1, Jörg Schwinger2, Ulysses S. Ninnemann3, Aurich Jeltsch-Thömmes4, Ingo Bethke1, Christoph Heinze1 5 1Geophysical Institute, University of Bergen and Bjerknes Centre for Climate Research, Bergen, 5007, Norway 2NORCE Norwegian Research Centre and Bjerknes Centre for Climate Research, Bergen, 5838, Norway 3Department of Earth Science, University of Bergen and Bjerknes Centre for Climate Research, Bergen, 5007, Norway 4Climate and Environmental Physics, Physics Institute and Oeschger Centre for Climate Change Research, 10 University of Bern, Bern, Switzerland Correspondence to: Anne L. Morée ([email protected]) Abstract. Although both physical and biological marine changes are required to explain the 100 ppm lower atmospheric pCO2 of the Last Glacial Maximum (LGM, ~21 ka) as compared to pre-industrial (PI) times, their exact contributions are debated. Proxies of past marine carbon cycling (such as δ13C) document these changes, and 15 thus provide constraints for quantifying the drivers of long-term carbon cycle variability. This modelling study explores the relative rolespresents a realization of the of physical and biological changes in the ocean needed to simulate an LGM ocean in satisfactory agreement with proxy data, and here especially δ13C. We prepared a PI and LGM equilibrium simulation using the ocean model state (NorESM-OC) with full biogeochemistry (including the carbon isotopes δ13C and radiocarbon) and dynamic sea ice. The modelled LGM-PI differences are evaluated 20 against a wide range of physical and biogeochemical proxy data, and show agreement for key aspects of the physical ocean state within the data uncertainties. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -
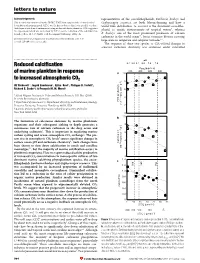
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics
CHAPTER SEVEN Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics Frans J. JorissenÃ, Christophe Fontanier and Ellen Thomas Contents 1. Introduction 263 1.1. General introduction 263 1.2. Historical overview of the use of benthic foraminiferal assemblages 266 1.3. Recent advances in benthic foraminiferal ecology 267 2. Benthic Foraminiferal Proxies: A State of the Art 271 2.1. Overview of proxy methods based on benthic foraminiferal assemblage data 271 2.2. Proxies of bottom water oxygenation 273 2.3. Paleoproductivity proxies 285 2.4. The water mass concept 301 2.5. Benthic foraminiferal faunas as indicators of current velocity 304 3. Conclusions 306 Acknowledgements 308 4. Appendix 1 308 References 313 1. Introduction 1.1. General Introduction The most popular proxies based on microfossil assemblage data produce a quanti- tative estimate of a physico-chemical target parameter, usually by applying a transfer function, calibrated on the basis of a large dataset of recent or core-top samples. Examples are planktonic foraminiferal estimates of sea surface temperature (Imbrie & Kipp, 1971) and reconstructions of sea ice coverage based on radiolarian (Lozano & Hays, 1976) or diatom assemblages (Crosta, Pichon, & Burckle, 1998). These à Corresponding author. Developments in Marine Geology, Volume 1 r 2007 Elsevier B.V. ISSN 1572-5480, DOI 10.1016/S1572-5480(07)01012-3 All rights reserved. 263 264 Frans J. Jorissen et al. methods are easy to use, apply empirical relationships that do not require a precise knowledge of the ecology of the organisms, and produce quantitative estimates that can be directly applied to reconstruct paleo-environments, and to test and tune global climate models. -

Marine Plankton Diatoms of the West Coast of North America
MARINE PLANKTON DIATOMS OF THE WEST COAST OF NORTH AMERICA BY EASTER E. CUPP UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES 1943 BULLETIN OF THE SCRIPPS INSTITUTION OF OCEANOGRAPHY OF THE UNIVERSITY OF CALIFORNIA LA JOLLA, CALIFORNIA EDITORS: H. U. SVERDRUP, R. H. FLEMING, L. H. MILLER, C. E. ZoBELL Volume 5, No.1, pp. 1-238, plates 1-5, 168 text figures Submitted by editors December 26,1940 Issued March 13, 1943 Price, $2.50 UNIVERSITY OF CALIFORNIA PRESS BERKELEY, CALIFORNIA _____________ CAMBRIDGE UNIVERSITY PRESS LONDON, ENGLAND [CONTRIBUTION FROM THE SCRIPPS INSTITUTION OF OCEANOGRAPHY, NEW SERIES, No. 190] PRINTED IN THE UNITED STATES OF AMERICA Taxonomy and taxonomic names change over time. The names and taxonomic scheme used in this work have not been updated from the original date of publication. The published literature on marine diatoms should be consulted to ensure the use of current and correct taxonomic names of diatoms. CONTENTS PAGE Introduction 1 General Discussion 2 Characteristics of Diatoms and Their Relationship to Other Classes of Algae 2 Structure of Diatoms 3 Frustule 3 Protoplast 13 Biology of Diatoms 16 Reproduction 16 Colony Formation and the Secretion of Mucus 20 Movement of Diatoms 20 Adaptations for Flotation 22 Occurrence and Distribution of Diatoms in the Ocean 22 Associations of Diatoms with Other Organisms 24 Physiology of Diatoms 26 Nutrition 26 Environmental Factors Limiting Phytoplankton Production and Populations 27 Importance of Diatoms as a Source of food in the Sea 29 Collection and Preparation of Diatoms for Examination 29 Preparation for Examination 30 Methods of Illustration 33 Classification 33 Key 34 Centricae 39 Pennatae 172 Literature Cited 209 Plates 223 Index to Genera and Species 235 MARINE PLANKTON DIATOMS OF THE WEST COAST OF NORTH AMERICA BY EASTER E.