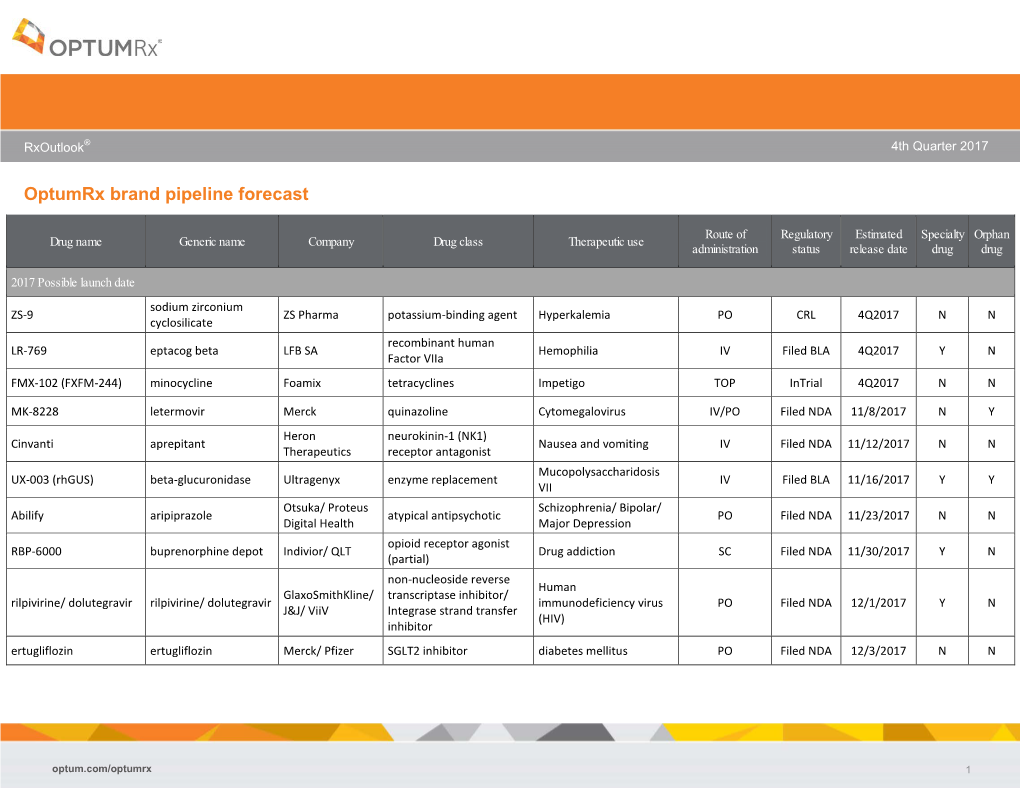
Optumrx Brand Pipeline Forecast
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upcoming Market Catalysts in Q4 2017
NEWS & ANALYSIS BIOBUSINESS BRIEFS MARKET WATCH Upcoming market catalysts in Q4 2017 Market catalysts in the fourth quarter of disease, and further results from a pivotal trial 2017 include top-line clinical trial results in Alzheimer disease are anticipated in late for tenapanor (developed by Ardelyx) for September. In light of the failure of other constipation-predominant irritable bowel 5HT6 receptor antagonists — including the syndrome (IBS-C) and intepirdine (developed recent failure of Lundbeck’s idalopirdine in by Axovant Sciences) for the treatment of several phase III trials in Alzheimer disease dementia with Lewy bodies, as well as the — the upcoming phase III data in Alzheimer expected approval of axicabtagene disease, along with the phase II data in ciloleucel (developed by Kite Pharma) in the dementia, will give important guidance United States for the treatment of relapsed/ to the future market opportunities for refractory aggressive B cell non-Hodgkin intepirdine. lymphoma (NHL). The Biologics License Application for Early in the quarter, Ardelyx expects Kite Pharma’s axicabtagene ciloleucel for the top-line results from T3MPO-2, a 6-month treatment of relapsed/refractory aggressive phase III study of tenapanor. This orally B cell NHL is under priority review, with a administered small molecule inhibits the Prescription Drug User Fee Act target action sodium–proton exchanger NHE3 in the date of 29 November 2017. This gastrointestinal tract, which increases the FDA-designated breakthrough therapy amount of sodium and fluid in the gut, consists of a patient’s peripheral blood thereby loosening stools. Results from a T lymphocytes that have been genetically previous 12-week phase III study known as engineered in vitro with chimeric antigen T3MPO-1 were statistically significant for receptors (CAR), enabling them to recognize the primary composite end point of response the tumour-expressed molecule CD19 rate, and seven out of eight secondary end after infusion back into the patient. -

The 1,3,5-Triazine Derivatives As Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment
International Journal of Molecular Sciences Article The 1,3,5-Triazine Derivatives as Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment Gniewomir Latacz 1 , Annamaria Lubelska 1, Magdalena Jastrz˛ebska-Wi˛esek 2, Anna Partyka 2, Małgorzata Anna Mar´c 1, Grzegorz Satała 3, Daria Wilczy ´nska 2, Magdalena Kota ´nska 4, Małgorzata Wi˛ecek 1, Katarzyna Kami ´nska 1, Anna Wesołowska 2, Katarzyna Kie´c-Kononowicz 1 and Jadwiga Handzlik 1,* 1 Department of Technology and Biotechnology of Drugs, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland 2 Department of Clinical Pharmacy, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland 3 Department of Medicinal Chemistry Institute of Pharmacology, Polish Academy of Science, Sm˛etna12, PL 31-343 Cracow, Poland 4 Department of Pharmacodynamics, Faculty of Pharmacy, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland * Correspondence: [email protected]; Tel.: +48-12-620-5580 Received: 27 May 2019; Accepted: 7 July 2019; Published: 12 July 2019 Abstract: Among serotonin receptors, the 5-HT6 subtype is the most controversial and the least known in the field of molecular mechanisms. The 5-HT6R ligands can be pivotal for innovative treatment of cognitive impairment, but none has reached pharmacological market, predominantly, due to insufficient “druglikeness” properties. Recently, 1,3,5-triazine-piperazine derivatives were identified as a new chemical family of potent 5-HT6R ligands. For the most active triazine 5-HT6R agents found (1–4), a wider binding profile and comprehensive in vitro evaluation of their drug-like parameters as well as behavioral studies and an influence on body mass in vivo were investigated within this work. -

Targeting Somatostatin Receptors: Preclinical Evaluation of Novel 18F-Fluoroethyltriazole-Tyr3-Octreotate Analogs for PET
Journal of Nuclear Medicine, published on August 18, 2011 as doi:10.2967/jnumed.111.088906 Targeting Somatostatin Receptors: Preclinical Evaluation of Novel 18F-Fluoroethyltriazole-Tyr3-Octreotate Analogs for PET Julius Leyton1, Lisa Iddon2, Meg Perumal1, Bard Indrevoll3, Matthias Glaser2, Edward Robins2, Andrew J.T. George4, Alan Cuthbertson3, Sajinder K. Luthra2, and Eric O. Aboagye1 1Comprehensive Cancer Imaging Center at Imperial College, Faculty of Medicine, Imperial College London, London, United Kingdom; 2MDx Discovery (part of GE Healthcare) at Hammersmith Imanet Ltd., Hammersmith Hospital, London, United Kingdom; 3GE Healthcare AS, Oslo, Norway; and 4Section of Immunobiology, Faculty of Medicine, Imperial College London, London, United Kingdom Key Words: somatostatin receptor; octreotide; 18F-fluoroethyl- The incidence and prevalence of gastroenteropancreatic triazole-Tyr3-octreotate; positron emission tomography; and neuroendocrine tumors has been increasing over the past 3 neuroendocrine decades. Because of high densities of somatostatin receptors J Nucl Med 2011; 52:1–8 (sstr)—mainly sstr-2—on the cell surface of these tumors, 111In- DOI: 10.2967/jnumed.111.088906 diethylenetriaminepentaacetic acid-octreotide scintigraphy has become an important part of clinical management. 18F-radio- labeled analogs with suitable pharmacokinetics would permit PET with more rapid clinical protocols. Methods: We compared the affinity in vitro and tissue pharmacokinetics by PET of 5 structurally related 19F/18F-fluoroethyltriazole-Tyr3-octreotate The incidence and prevalence of gastroenteropancreatic (FET-TOCA) analogs: FET-G-polyethylene glycol (PEG)-TOCA, neuroendocrine tumors (GEP-NETs) has increased signifi- FETE-PEG-TOCA, FET-G-TOCA, FETE-TOCA, and FET-bAG- cantly over the past 3 decades (1). The most common site TOCA to the recently described 18F-aluminum fluoride NOTA- of primary GEP-NETs is the gastrointestinal tract (60%). -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Say on Pay Results (As of September 5)
THIS REPORT CAN BE ACCESSED AT HTTP://WWW.SEMLERBROSSY.COM/SAYONPAY NOTE: THIS WILL BE OUR FINAL SAY ON PAY UPDATE FOR 2012. WE WILL ISSUE A FULL REPORT PROVIDING RESULTS FOR THE ENTIRE 2012 PROXY SEASON IN JANUARY 2013. PLEASE CONTINUE TO VISIT OUR SAY ON PAY BLOG FOR UPDATES. SAY ON PAY RESULTS 2012 RUSSELL 3000 SEPTEMBER 5 2012 SAY ON PAY RESULTS: RUSSELL 3000 SHAREHOLDER VOTING SUMMARY OF FINDINGS 2012 Vote Results (n=2,025) 2 The majority of companies continue to pass Say on Pay in 2012 with substantial shareholder support: — 1,466 companies (72%) passed with over 90% support — 381 companies (19%) passed with between 70% and 90% support — 125 companies (6%) passed with between 50% and 70% support — 53 companies (2.6%) in the Russell 3000 have failed Vote of the Week McKesson received a vote of 62%, a decline of 8% from 2011, amidst criticism from shareholders and 3 their advisors over high relative CEO pay and retirement benefits Vote Results by Industry Health Care companies have received proportionally less support than other industries, while 4 Consumer Staple and Financial companies have received the most support Vote Results and Market Value 5 There does not appear to be a strong correlation between a company’s market value and Say on Pay vote result How Vote Results Changed in 2012 6 Companies below 70% in 2011 have generally received increased vote support in 2012: — 26 of 30 companies that failed in 2011 have passed in 2012 — Companies between 50‐70% in 2011 have improved by an average of 13% in 2012 Vote results for companies -

G Protein-Coupled Receptors As Therapeutic Targets for Multiple Sclerosis
npg GPCRs as therapeutic targets for MS Cell Research (2012) 22:1108-1128. 1108 © 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 npg REVIEW www.nature.com/cr G protein-coupled receptors as therapeutic targets for multiple sclerosis Changsheng Du1, Xin Xie1, 2 1Laboratory of Receptor-Based BioMedicine, Shanghai Key Laboratory of Signaling and Disease Research, School of Life Sci- ences and Technology, Tongji University, Shanghai 200092, China; 2State Key Laboratory of Drug Research, the National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 189 Guo Shou Jing Road, Pudong New District, Shanghai 201203, China G protein-coupled receptors (GPCRs) mediate most of our physiological responses to hormones, neurotransmit- ters and environmental stimulants. They are considered as the most successful therapeutic targets for a broad spec- trum of diseases. Multiple sclerosis (MS) is an inflammatory disease that is characterized by immune-mediated de- myelination and degeneration of the central nervous system (CNS). It is the leading cause of non-traumatic disability in young adults. Great progress has been made over the past few decades in understanding the pathogenesis of MS. Numerous data from animal and clinical studies indicate that many GPCRs are critically involved in various aspects of MS pathogenesis, including antigen presentation, cytokine production, T-cell differentiation, T-cell proliferation, T-cell invasion, etc. In this review, we summarize the recent findings regarding the expression or functional changes of GPCRs in MS patients or animal models, and the influences of GPCRs on disease severity upon genetic or phar- macological manipulations. -

Alshaer Danah Mahdi 2020.Pdf (14.70Mb)
Synthesis and physiochemical characterization of new siderophore- inspired peptide-chelators with 1- hydroxypridine-2-one (1,2-HOPO) Thesis Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy by Danah Mahdi AlShaer 2020 Supervisor: Prof. Beatriz Garcia de la Torre Co-supervisor: Prof. Fernando Albericio Synthesis and physiochemical characterization of new siderophore-inspired peptide• chelators with 1-hydrnxypridine-2-one (1,2-JB[OPO) 217078895 Danah Mahdi AllSlh.aer A thesis submitted to the School of Health Sciences, College of Health Sciences, University of KwaZulu-Natal, Westville, for the degree of Doctor of Philosophy by research in Pharmaceutical Chemistry. This is the thesis in which the chapters are written as a set of discrete research publications that have followed each journal's format with an overall introduction and final summary. These chapters have been published in internationally recognized, peer-reviewed journals. This is to certify that the contents of this thesis are the original research work of Mrs Danah Mahdi AllSllnaer, carried out under our supervision at the Peptide Sciences Laboratory, Westville campus, University of KwaZulu-Natal, Durban, South Africa. Supervisor: --"-:I-----::... Date: gth December 2020 Date: 8th December 2020 As the candidate's supervisors we agree to the submission of this thesis Table of Contents Abstract……………..…………………………………………………………………..……1 Declaration 1: Plagiarism.……………………………………………………….…………..2 Declaration 2: Publications ….………………………………………………….…...….…..3 Acknowledgment………………………………………..………………...…………….…..5 Aim and objectives……….….……………………………………………………………...6 Chapter 1: (Introduction) Hydroxamate Siderophores: Natural Occurrence, Chemical Synthesis, Iron Binding Affinity and Use as Trojan Horses Against ………..……….. 7 Reprint………………………………………………………………………………………8 Chapter 2: Solid-phase synthesis of peptides containing 1-Hydroxypyridine-2-one (1,2-HOPO) …………………………………………………….………………………. -

Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future
International Journal of Molecular Sciences Review Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future Anna Kathrin Stueven 1, Antonin Kayser 1, Christoph Wetz 2, Holger Amthauer 2, Alexander Wree 1, Frank Tacke 1, Bertram Wiedenmann 1, Christoph Roderburg 1,* and Henning Jann 1 1 Charité, Campus Virchow Klinikum and Charité, Campus Mitte, Department of Hepatology and Gastroenterology, Universitätsmedizin Berlin, 10117 Berlin, Germany; [email protected] (A.K.S.); [email protected] (A.K.); [email protected] (A.W.); [email protected] (F.T.); [email protected] (B.W.); [email protected] (H.J.) 2 Charité, Campus Virchow Klinikum and Charité, Campus Mitte, Department of Nuclear Medicine, Universitätsmedizin Berlin, 10117 Berlin, Germany; [email protected] (C.W.); [email protected] (H.A.) * Correspondence: [email protected]; Tel.: +49-30-450-553022 Received: 3 May 2019; Accepted: 19 June 2019; Published: 22 June 2019 Abstract: In recent decades, the incidence of neuroendocrine tumors (NETs) has steadily increased. Due to the slow-growing nature of these tumors and the lack of early symptoms, most cases are diagnosed at advanced stages, when curative treatment options are no longer available. Prognosis and survival of patients with NETs are determined by the location of the primary lesion, biochemical functional status, differentiation, initial staging, and response to treatment. Somatostatin analogue (SSA) therapy has been a mainstay of antisecretory therapy in functioning neuroendocrine tumors, which cause various clinical symptoms depending on hormonal hypersecretion. Beyond symptomatic management, recent research demonstrates that SSAs exert antiproliferative effects and inhibit tumor growth via the somatostatin receptor 2 (SSTR2). -

Current Advances in Allosteric Modulation of Muscarinic Receptors
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 18 January 2020 Peer-reviewed version available at Biomolecules 2020, 10, 325; doi:10.3390/biom10020325 Review Current Advances in Allosteric Modulation of Muscarinic Receptors Jan Jakubik 1* and Esam E. El-Fakahany 2* 1 Department of Neurochemistry, Institute of Physiology CAS, Prague, Czech Republic; [email protected] 2 Department of Experimental and Clinical Pharmacology, University of Minnesota College of Pharmacy, Minneapolis, MN, USA; [email protected] * Correspondence: [email protected]; [email protected] Abstract: Allosteric modulators are ligands that bind to a site on the receptor that is spatially separated from the orthosteric binding site for the endogenous neurotransmitter. Allosteric modulators modulate the binding affinity, potency and efficacy of orthosteric ligands. Muscarinic acetylcholine receptors are prototypical allosterically-modulated G-protein-coupled receptors. They are a potential therapeutic target for the treatment of psychiatric, neurologic and internal diseases like schizophrenia, Alzheimer’s disease, Huntington disease, type 2 diabetes or chronic pulmonary obstruction. Here we review progress made during the last decade in our understanding of their mechanisms of binding, allosteric modulation and in vivo actions of in order to understand the translational impact of studying this important class of pharmacological agents. We overview newly developed allosteric modulators of muscarinic receptors as well as new spin-off ideas like bitopic ligands combining allosteric and orthosteric moieties and photo-switchable ligands based on bitopic agents. Keywords: acetylcholine; muscarinic receptors; allosteric modulation 1. Introduction Slow metabotropic responses to acetylcholine are mediated by muscarinic receptors. Five distinct subtypes of muscarinic acetylcholine receptors (M1-M5) have been identified in the human genome[1]. -

Fidelity® Total Market Index Fund
Quarterly Holdings Report for Fidelity® Total Market Index Fund May 31, 2021 STI-QTLY-0721 1.816022.116 Schedule of Investments May 31, 2021 (Unaudited) Showing Percentage of Net Assets Common Stocks – 99.3% Shares Value Shares Value COMMUNICATION SERVICES – 10.1% World Wrestling Entertainment, Inc. Class A (b) 76,178 $ 4,253,780 Diversified Telecommunication Services – 1.1% Zynga, Inc. (a) 1,573,367 17,055,298 Alaska Communication Systems Group, Inc. 95,774 $ 317,970 1,211,987,366 Anterix, Inc. (a) (b) 16,962 838,941 Interactive Media & Services – 5.6% AT&T, Inc. 11,060,871 325,521,434 Alphabet, Inc.: ATN International, Inc. 17,036 805,292 Class A (a) 466,301 1,099,001,512 Bandwidth, Inc. (a) (b) 34,033 4,025,764 Class C (a) 446,972 1,077,899,796 Cincinnati Bell, Inc. (a) 84,225 1,297,065 ANGI Homeservices, Inc. Class A (a) 120,975 1,715,426 Cogent Communications Group, Inc. (b) 66,520 5,028,912 Autoweb, Inc. (a) (b) 6,653 19,028 Consolidated Communications Holdings, Inc. (a) 110,609 1,035,300 Bumble, Inc. 77,109 3,679,641 Globalstar, Inc. (a) (b) 1,067,098 1,707,357 CarGurus, Inc. Class A (a) 136,717 3,858,154 IDT Corp. Class B (a) (b) 31,682 914,343 Cars.com, Inc. (a) 110,752 1,618,087 Iridium Communications, Inc. (a) 186,035 7,108,397 DHI Group, Inc. (a) (b) 99,689 319,005 Liberty Global PLC: Eventbrite, Inc. (a) 114,588 2,326,136 Class A (a) 196,087 5,355,136 EverQuote, Inc. -

Japanese Guidelines for Adult Asthma 2020*
Allergology International xxx (xxxx) xxx Contents lists available at ScienceDirect Allergology International journal homepage: http://www.elsevier.com/locate/alit Invited Review Article Japanese guidelines for adult asthma 2020* * Yoichi Nakamura a, , Jun Tamaoki b, Hiroyuki Nagase c, Masao Yamaguchi d, Takahiko Horiguchi e, Soichiro Hozawa f, Masakazu Ichinose g, Takashi Iwanaga h, Rieko Kondo e, Makoto Nagata i, Akihito Yokoyama j, Yuji Tohda h, The Japanese Society of Allergology a Medical Center for Allergic and Immune Diseases, Yokohama City Minato Red Cross Hospital, Yokohama, Japan b First Department of Medicine, Tokyo Women's Medical University, Tokyo, Japan c Division of Respiratory Medicine and Allergology, Department of Medicine, Teikyo University School of Medicine, Tokyo, Japan d Third Department of Medicine, Teikyo University Chiba Medical Center, Chiba, Japan e Department of Respiratory Medicine, Fujita Health University Bantane Hospital, Nagoya, Japan f Hiroshima Allergy and Respiratory Clinic, Hiroshima, Japan g Department of Respiratory Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan h Department of Respiratory Medicine and Allergology, Kinki University Faculty of Medicine, Osaka, Japan i Department of Respiratory Medicine, Saitama Medical University Hospital, Saitama, Japan j Department of Hematology and Respiratory Medicine, Kochi University, Kochi, Japan article info abstract Article history: Bronchial asthma is characterized by chronic airway inflammation, which manifests clinically as variable Received 9 June 2020 airway narrowing (wheezes and dyspnea) and cough. Long-standing asthma may induce airway Available online xxx remodeling and become intractable. The prevalence of asthma has increased; however, the number of patients who die from it has decreased (1.3 per 100,000 patients in 2018). -

Drug Candidates in Clinical Trials for Alzheimer's Disease
Hung and Fu Journal of Biomedical Science (2017) 24:47 DOI 10.1186/s12929-017-0355-7 REVIEW Open Access Drug candidates in clinical trials for Alzheimer’s disease Shih-Ya Hung1,2 and Wen-Mei Fu3* Abstract Alzheimer’s disease (AD) is a major form of senile dementia, characterized by progressive memory and neuronal loss combined with cognitive impairment. AD is the most common neurodegenerative disease worldwide, affecting one-fifth of those aged over 85 years. Recent therapeutic approaches have been strongly influenced by five neuropathological hallmarks of AD: acetylcholine deficiency, glutamate excitotoxicity, extracellular deposition of amyloid-β (Aβ plague), formation of intraneuronal neurofibrillary tangles (NTFs), and neuroinflammation. The lowered concentrations of acetylcholine (ACh) in AD result in a progressive and significant loss of cognitive and behavioral function. Current AD medications, memantine and acetylcholinesterase inhibitors (AChEIs) alleviate some of these symptoms by enhancing cholinergic signaling, but they are not curative. Since 2003, no new drugs have been approved for the treatment of AD. This article focuses on the current research in clinical trials targeting the neuropathological findings of AD including acetylcholine response, glutamate transmission, Aβ clearance, tau protein deposits, and neuroinflammation. These investigations include acetylcholinesterase inhibitors, agonists and antagonists of neurotransmitter receptors, β-secretase (BACE) or γ-secretase inhibitors, vaccines or antibodies targeting Aβ clearance or tau protein, as well as anti-inflammation compounds. Ongoing Phase III clinical trials via passive immunotherapy against Aβ peptides (crenezumab, gantenerumab, and aducanumab) seem to be promising. Using small molecules blocking 5-HT6 serotonin receptor (intepirdine), inhibiting BACE activity (E2609, AZD3293, and verubecestat), or reducing tau aggregation (TRx0237) are also currently in Phase III clinical trials.