Separation of Biochemical Buffering Agents Using Multi-Mode Liquid
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Thermodynamic Quantities for the Ionization Reactions of Buffers
Thermodynamic Quantities for the Ionization Reactions of Buffers Robert N. Goldberg,a… Nand Kishore,b… and Rebecca M. Lennen Biotechnology Division, National Institute of Standards and Technology, Gaithersburg, Maryland 20899 ͑Received 21 June 2001; accepted 16 September 2001; published 24 April 2002͒ This review contains selected values of thermodynamic quantities for the aqueous ionization reactions of 64 buffers, many of which are used in biological research. Since the aim is to be able to predict values of the ionization constant at temperatures not too far from ambient, the thermodynamic quantities which are tabulated are the pK, standard ⌬ ؠ ⌬ molar Gibbs energy rG , standard molar enthalpy rH°, and standard molar heat ca- ؠ ⌬ pacity change rC p for each of the ionization reactions at the temperature T ϭ298.15 K and the pressure pϭ0.1 MPa. The standard state is the hypothetical ideal solution of unit molality. The chemical name͑s͒ and CAS registry number, structure, empirical formula, and molecular weight are given for each buffer considered herein. The selection of the values of the thermodynamic quantities for each buffer is discussed. © 2002 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. Key words: buffers; enthalpy; equilibrium constant; evaluated data; Gibbs free energy; heat capacity; ionization; pK; thermodynamic properties. Contents 7.19. CAPSO.............................. 263 7.20. Carbonate............................ 264 7.21. CHES............................... 264 1. Introduction................................ 232 7.22. Citrate............................... 265 2. Thermodynamic Background.................. 233 7.23. L-Cysteine............................ 268 3. Presentation of Data......................... 234 7.24. Diethanolamine........................ 270 4. Evaluation of Data. ........................ 235 7.25. Diglycolate.......................... -

Buffers a Guide for the Preparation and Use of Buffers in Biological Systems Calbiochem® Buffers a Guide for the Preparation and Use of Buffers in Biological Systems
Buffers A guide for the preparation and use of buffers in biological systems Calbiochem® Buffers A guide for the preparation and use of buffers in biological systems Chandra Mohan, Ph.D. EMD, San Diego, California © EMD, an affiliate of Merck KGaA, Darmstadt, Germany. All rights reserved. A word to our valued customers We are pleased to present to you the newest edition of Buffers: A Guide for the Preparation and Use of Buffers in Biological Systems. This practical resource has been especially revamped for use by researchers in the biological sciences. This publication is a part of our continuing commitment to provide useful product information and exceptional service to you, our customers. You will find this booklet a highly useful resource, whether you are just beginning your research work or training the newest researchers in your laboratory. Over the past several years, EMD Biosciences has clearly emerged as a world leader in providing highly innovative products for your research needs in Signal Transduction, including the areas of Cancer Biology, Alzheimer’s Disease, Diabetes, Hypertension, Inflammation, and Apoptosis. Please call us today for a free copy of our LATEST Catalog that includes tools for signal transduction and life science research. If you have used our products in the past, we thank you for your support and confidence in our products, and if you are just beginning your research career, please call us and give us the opportunity to demonstrate our exceptional customer and technical service. Corrine Fetherston Sr. Director, Marketing ii Table of Contents: Why does Calbiochem® Biochemicals Publish a Booklet on Buffers? . -

Biological Buffers and Ultra Pure Reagents
Biological Buffers and Ultra Pure Reagents Are MP Buffers in your corner? One Call. One Source. A World of Ultra Pure Biochemicals. www.mpbio.com Theoretical Considerations Since buffers are essential for controlling the pH in many Since, under equilibrium conditions, the rates of dissociation and biological and biochemical reactions, it is important to have a association must be equal, they may be expressed as: basic understanding of how buffers control the hydrogen ion concentration. Although a lengthy, detailed discussion is impractical, + - k1 (HAc) = k2 (H ) (Ac ) some explanation of the buffering phenomena is important. Or Let us begin with a discussion of the equilibrium constant (K) for + - weak acids and bases. Acids and bases which do not completely k1 = (H ) (Ac ) dissociate in solution, but instead exist as an equilibrium mixture of k (HAc) undissociated and dissociated species, are termed weak acids and 2 bases. The most common example of a weak acid is acetic acid. If we now let k1/k2 = Ka , the equilibrium constant, the equilibrium In solution, acetic acid exists as an equilibrium mixture of acetate expression becomes: ions, hydrogen ions, and undissociated acetic acid. The equilibrium between these species may be expressed as follows: + - Ka = (H ) (Ac ) k1 (HAc) + - HAc ⇌ H + Ac which may be rearranged to express the hydrogen ion concentration k2 in terms of the equilibrium constant and the concentrations of undissociated acetic acid and acetate ions as follows: where k1 is the dissociation rate constant of acetic acid to acetate and hydrogen ions and k is the association rate constant of the ion 2 (H+) = K (HAc) species to form acetic acid. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0030594 A1 ABRAMS Et Al
US 2016.0030594A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0030594 A1 ABRAMS et al. (43) Pub. Date: Feb. 4, 2016 (54) ANTIBODY DRUG CONJUGATES Related U.S. Application Data (71) Applicants: Tinya ABRAMS, Acton, MA (US); (60) Provisional application No. 61/793,641, filed on Mar. Steven COHEN, San Diego, CA (US); 15, 2013. Christie P. FANTON, Oakland, CA EEEE): Publication Classification Kathy MILLER, San Francisco, CA (51) Int. Cl (US); Siew Ho SCHLEYER, El Cerrito, we CA (US); Kathrin Ulrike g 7. :08: TISSOT-DAGUETTE, Planegg (DE) A647/12 (2006.01) (72) Inventors: Tinya ABRAMS, Acton, MA (US); A647/26 (2006.01) Steven COHEN, San Diego, CA (US); A 6LX3/537 (2006.01) Christie P. FANTON, Oakland, CA A6II 45/06 (2006.01) (US); Catrin FINNER, Neuried (DE); (52) U.S. Cl. Thomas HUBER, Allschwil (CH): CPC. A61 K47/48561 (2013.01); A61K 47/48384 Kathy MILLER, San Francisco, CA (2013.01); A61 K3I/537 (2013.01); A61 K (US); Siew Ho SCHLEYER, El Cerrito, 45/06 (2013.01); A61 K47/12 (2013.01); A61 K CA (US); Kathrin Ulrike 47/26 (2013.01); A61 K47/4863 (2013.01); TISSOT-DAGUETTE, Planegg (DE) A61K47/48592 (2013.01); A61K 47/48615 (2013.01); C07K 16/32 (2013.01); C07K (21) Appl. No.: 14/774,512 231 7/565 (2013.01); C07K 231 7/24 (2013.01) (86). PCT No.: PCT/US14/24597 The present invention relates to anti-cKIT antibodies, anti S371 (c)(1), body fragments, antibody drug conjugates, and their uses for (2) Date: Sep.10, 2015 the treatment of cancer. -

WO 2018/185618 Al 11 October 2018 (11.10.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/185618 Al 11 October 2018 (11.10.2018) W !P O PCT (51) International Patent Classification: (74) Agent: NOVARTIS AG; Lichtstrasse 35, 4056 Basel A61K 47/68 (2017.01) A61P 35/00 (2006.01) (CH). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/IB20 18/05215 1 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, (22) International Filing Date: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, 28 March 2018 (28.03.2018) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (25) Filing Language: English HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (26) Publication Langi English MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (30) Priority Data: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/480,972 03 April 2017 (03.04.2017) US SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: NOVARTIS AG [CH/CH]; Lichtstrasse 35, 4056 Basel (CH). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors: ANTONAKOS, Brandon Peter; Novartis In GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, stitutes for BioMedical Research, Inc., 250 Massachu UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, setts Avenue, Cambridge, Massachusetts 02139 (US). -

Biological Buffers
AppliCations No.2 Biological Buffers Many biochemical processes are markedly impaired by even small changes in the con centrations of free H+ ions. It is therefore usually necessary to stabilise the H+ concentration in vitro by adding a suitable buffer to the medium, without, however, affecting the functioning of the system under investigation. A buffer keeps the pH of a solution constant by taking up protons that are released during reactions, or by r eleasing protons when they are consumed by reactions. This handout summarizes the most commonly used buffer substances and respec- tive physical and chemical properties. Keywords Practical Tips – Preparing Buffer Solutions chemical properties Recommendations for the setting of the pH value of a buffer and storage conditions usefull pH range 1. Temperature Depending on the buffer substance, its pH may vary with temperature. It is therefore advisable, as far as possible, to set the pH buffer preparation at the working temperature to be used for the investigation. For instance the physiological pH value for most mammalian cells at 37°C is between 7.0 and 7.5. The temperature dependence of a buffer system is expressed as d(pKa)/dT, which describes the change of the pKa at an increase of temperature by 1°C. 2. Titration (i) Generally, the pH value is set using NaOH/KOH or HCl. Slow addition of a strong acid or base whilst stirring vigorously avoids local high concentrations of H+ or OH– ions. If this is not done, the buffer substances may undergo chemical changes that inactivate them or modify them so that they have an inhibitory action (Ellis & Morrison 1982). -

Department of Paediatrics CHAIR/CHIEF’S REPORT
ANNUAL REPORT 2015-16 Department of Paediatrics CHAIR/CHIEF’S REPORT 3. clinical care is enriched by the patient safety and quality strategies; choosing wisely initiatives; greater accountability and more. And now some direct thanks: • to the members of the Paediatrics Executive for their unwavering support and effort: Rayfel Schneider (education), Meredith Irwin (research), Jeremy Friedman (clinical), Marilyn Monk (hospital operations) and Ben Vozzolo (departmental operations); • to the division chiefs and their training program directors for keeping us heading forward; the end of June 2016, my ten year term as Chair At of the Department of Paediatrics at the University • to the Dean, Vice-Deans, Associate Deans of the TABLE OF of Toronto and Paediatrician-in-Chief at SickKids came Medical School, so ably led by Dean Trevor Young; to an end, ten years being the rule at these two great SickKids Senior Executive team, led first Mary Jo institutions. And completely appropriately too, since all Haddad and now Mike Apkon. CONTENTS institutions need periods of renewal and reflection in order to steam ahead. I have no doubt that Ronald Cohn who In the past 10 years we recruited over 100 new faculty, has assumed these roles is an outstanding choice and promoted to Full or Associate Professor 154 members 3 CHAIR/CHIEF’S REPORT 146 INFECTIOUS DISEASES will move the department ever forwards. of the faculty. I believe our commitment to excellence has served us very well indeed and will continue to do so. CARDIOLOGY NEONATOLOGY 12 158 I would personally wish to thank all members of the department, whether at SickKids or in other UofT-affiliated It has been a joy and a privilege to have been in these 30 CLINICAL GENETICS & METABOLICS 168 NEPHROLOGY institutions, for their enormous contributions. -

Tocris製品30%Offキャンペーン価格表(2021/7/5~2021/8/31)
TOCRIS製品30%OFFキャンペーン価格表(2021/7/5~2021/8/31) (メーカーコード順) 希望納⼊価格 キャンペーン価格 コードNo.メーカーコード 英名 容量 (円) (円) 537-31171 0101/100 DL-2-Amino-4-phosphonobutyric Acid [DL-AP4] 100mg 24,000 16,800 - 0102/10 D(-)-2-Amino-4-phosphonobutyric Acid [D-AP4] 10mg 52,000 36,400 - 0102/50 D(-)-2-Amino-4-phosphonobutyric Acid [D-AP4] 50mg 222,000 155,400 - 0103/1 L(+)-2-Amino-4-phosphonobutyric Acid [L-AP4] 1mg 18,000 12,600 531-26804 0103/10 L(+)-2-Amino-4-phosphonobutyric Acid [L-AP4] 10mg 46,000 32,200 533-26803 0103/50 L(+)-2-Amino-4-phosphonobutyric Acid [L-AP4] 50mg 203,000 142,100 - 0104/10 DL-AP7 10mg 30,000 21,000 - 0104/50 DL-AP7 50mg 120,000 84,000 - 0105/10 DL-AP5 10mg 20,000 14,000 530-57943 0105/50 DL-AP5 50mg 81,000 56,700 - 0106/1 D-AP5 1mg 15,000 10,500 531-26843 0106/10 D-AP5 10mg 39,000 27,300 535-26846 0106/100 D-AP5 100mg 235,000 164,500 539-26844 0106/50 D-AP5 50mg 174,000 121,800 - 0107/10 L-AP5 10mg 54,000 37,800 - 0107/50 L-AP5 50mg 235,000 164,500 514-20993 0109/10 (-)-Bicuculline methobromide 10mg 28,000 19,600 518-20991 0109/50 (-)-Bicuculline methobromide 50mg 126,000 88,200 - 0111/1 Dihydrokainic acid 1mg 17,000 11,900 - 0111/10 Dihydrokainic acid 10mg 42,000 29,400 - 0111/50 Dihydrokainic acid 50mg 189,000 132,300 532-28291 0112/50 gamma-D-Glutamylglycine 50mg 38,000 26,600 539-26861 0114/50 N-Methyl-D-aspartic Acid [NMDA] 50mg 24,000 16,800 535-26863 0114/500 N-Methyl-D-aspartic Acid [NMDA] 500mg 100,000 70,000 533-31151 0125/100 DL-AP3 100mg 30,000 21,000 512-21011 0130/50 (+)-Bicuculline 50mg 47,000 32,900 535-57954 0131/10 (-)-Bicuculline -

Biological Buffers and Ultra Pure Reagents
Biological Buffers and Ultra Pure Reagents One Call. One Source. A World of Ultra Pure Biochemicals. Are MP Buffers in your corner? Find it at fishersci.com Theoretical Considerations Since buffers are essential for controlling the pH in many Since, under equilibrium conditions, the rates of dissociation and biological and biochemical reactions, it is important to have a association must be equal, they may be expressed as: basic understanding of how buffers control the hydrogen ion concentration. Although a lengthy, detailed discussion is impractical, + - k1 (HAc) = k2 (H ) (Ac ) some explanation of the buffering phenomena is important. Or Let us begin with a discussion of the equilibrium constant (K) for + - weak acids and bases. Acids and bases which do not completely k1 = (H ) (Ac ) dissociate in solution, but instead exist as an equilibrium mixture of k (HAc) undissociated and dissociated species, are termed weak acids and 2 bases. The most common example of a weak acid is acetic acid. If we now let k1/k2 = Ka , the equilibrium constant, the equilibrium In solution, acetic acid exists as an equilibrium mixture of acetate expression becomes: ions, hydrogen ions, and undissociated acetic acid. The equilibrium between these species may be expressed as follows: + - Ka = (H ) (Ac ) k1 (HAc) HAc H+ + Ac- ⇌ which may be rearranged to express the hydrogen ion concentration k2 in terms of the equilibrium constant and the concentrations of undissociated acetic acid and acetate ions as follows: where k1 is the dissociation rate constant of acetic acid to acetate and hydrogen ions and k is the association rate constant of the ion 2 (H+) = K (HAc) species to form acetic acid. -
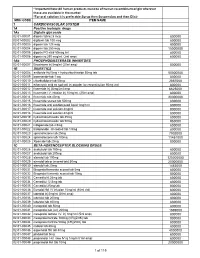
National Master List of Drugs and Lab Reagents
* Important Note:All human products must be of human recombinant origin wherever these are available in the market *For oral solution it is preferable:Syrup then Suspension and then Elixir MOH CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1Aa Digtalis glycoside 02-01-00001 digoxin tab 62.5 mcg 800000 02-01-00002 digitoxin tab 100 mcg 800000 02-01-00003 digoxin tab 125 mcg 800000 02-01-00004 digoxin tab 250 mcg 15000000 02-01-00005 digoxin PG elixir 50mcg /ml 800000 02-01-00006 digoxin inj 250 mcg/ml, (2ml amp) 800000 1Ab PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone inj 5mg/ml (20ml amp) 800000 1B DIURETICS 02-01-00008 amiloride Hcl 5mg + hydrochlorthiazide 50mg tab 50000000 02-01-00009 bumetanide tab 1 mg 800000 02-01-00010 chlorthalidone tab 50mg 2867000 02-01-00011 ethacrynic acid as sod.salt inj powder for reconstitution 50mg vial 800000 02-01-00012 frusemide inj 20mg/2ml amp 6625000 02-01-00013 frusemide I.V. infusion inj 10mg/ml, (25ml amp) 800000 02-01-00014 frusemide tab 40mg 20000000 02-01-00015 frusemide scored tab 500mg 800000 02-01-00016 frusemide oral solution pead liquid 1mg/1ml 800000 02-01-00017 frusemide oral solution 4mg/ml 800000 02-01-00018 frusemide oral solution 8mg/ml 800000 02-01-00019 hydrochlorothiazide tab 25mg 800000 02-01-00020 hydrochlorothiazide tab 50mg 950000 02-01-00021 indapamide tab 2.5mg 800000 02-01-00022 Indapamide s/r coated tab 1.5mg 800000 02-01-00023 spironolactone tab 25mg 7902000 02-01-00024 spironolactone tab 100mg 11451000 02-01-00025 Xipamide tab 20mg 800000 1C -

Semi-Bulk Products CAS ACACIA POWDER 9000-01-5 ACCOFLOC A
Semi-Bulk Products CAS ACACIA POWDER 9000-01-5 ACCOFLOC A 110H NA ACENAPHTHENE 83-32-9 ACES 7365-82-4 ACETAL 105-57-7 ACETALDEHYDE OXIME 107-29-9 ACETAMIDE 60-35-5 ACETAMIDE OXIME 22059-22-9 ACETAMIDINE HYDROCHLORIDE 124-42-5 5-ACETAMIDO-2-BROMOBENZOIC ACID 22921-67-1 ACETANILIDE 103-84-4 ACETOACETANILIDE 102-01-2 ACETOBROMO-A-D-GLUCOSE 572-09-8 ACETOGUANAMINE 542-02-9 ACETOHYDROXAMIC ACID 546-88-3 ACETOIN 513-86-0 ACETONITRILE 75-05-8 ACETOPHENONE 98-86-2 2-ACETOXYACETOPHENONE 7250-94-4 ACETOXYACETYL CHLORIDE 13831-31-7 4-ACETOXYBENZALDEHYDE 878-00-2 (3S,4R)-4-ACETOXY-3(R)-(T-BUTYLDIMETHYLSILYOXYETHYL)-2-AZETIDIONE 76855-69-1 ACETOXY ISOBUTYRYL BROMIDE 40635-67-4 ACETURIC ACID 543-24-8 ACETYLACETONE 123-54-6 3-ACETYLBENZONITRILE 6136-68-1 4-ACETYLBENZONITRILE 1443-80-7 2-ACETYL-5-BROMOTHIOPHENE 5370-25-2 N-ACETYL-Y-BUTYROLACTONE 517-23-7 1-ACETYL-4-(2-CHLOROETHYL)PIPERAZINE HYDROCHLORIDE 92928-18-2 N-ACETYL-L-CYSTEINE 616-91-1 3-O-ACETYL-1,2,5,6-DI-O-ISOPROPYLIDENE-A-D-GLUCOFURANOSE 16713-80-7 ACETYLENE DICARBOXYLIC ACID MONO POTASSIUM SALT 928-04-1 N-ACETYL-D-GALACTOSAMINE 14215-68-0 N-ACETYL-D-GLUCOSAMINE 7512-17-6 N-ACETYL-D-GLUTAMIC ACID 19146-55-5 N-ACETYL-L-GLUTAMIC ACID 1188-37-0 N-ACETYL-L-GLUTAMINE 2490-97-3 N-ACETYLGLYCINE 543-24-8 1-ACETYLGUANIDINE 5699-40-1 N-ACETYL-4(4-HYDROXYPHENYL) PIPERAZINE 67914-60-7 N-EPSILON-ACETYL-L-LYSINE 692-04-6 N-ACETYL-DL-METHIONINE 1115-47-5 2-ACETYL-6-METHOXYNAPHTHALENE 3900-45-6 N-ACETYLNEURAMINIC ACID 131-48-6 4-ACETYLPHENOXYACETIC ACID 1878-81-5 N-ACETYLPHENYL HYDRAZINE 114-83-0 2-ACETYLPYRIDINE -

Sept 2015 Core Reagents Please Contact Us for Details
*Product Spec could be varied by Lot #. Phone 858-452-9925 Email [email protected] Sept 2015 Core Reagents Please contact us for details. Web www.agscientific.com Product Specification Price Comparison In Stock Pro # Product Name CAS # Purity Ag Price Vendor S Price Bulk/OEM/Custom Available A-1018 AEBSF HCl 30827-99-7 ≥97% (HPLC) 250 mg- $142.00 100 mg- $121.00 50 mg/ 250 mg/ 1 g A-1020 A23187 52665-69-7 >99% (TLC) 10 mg- $117 10 mg- $136 5 mg/ 10 mg/ 50 mg A-1022 Actinomycin D 50-76-0 > 99% (HPLC) 10 mg- $115 10 mg- $174 5 mg/ 10mg/ 25 mg/ 100 mg A-1049 Anisomycin 22862-76-6 98% (HPLC) 100 mg- $170 100 mg- $406 10 mg/ 50 mg/ 100 mg/ 500 mg A-1128 A23187, Mixed Ca-mg Salt 98% (TLC) 10 mg - $117 10 mg - $186 10 mg A-1256 17-AAg 75747-14-7 >99% (HPLC) 500 μg- $36 500 μg- $195 500 μg / 1 mg/ 5 mg A-1298 17-DMAG 467214-20-6 >98% (TLC) 1 mg- $50 1 mg- $275 1 mg/ 5 mg A-1716 AICAR 2627-69-2 >98% (HPLC) 50 mg- $105.00 25mg- $191.50 50 mg A-2172 5-Azacytidine 320-67-2 >98% (TLC) 250 mg- $135 250 mg- $90 50 mg/ 250 mg A-2370 2-APB 524-95-8 97% 100 mg- $55.00 25 mg- $206.00 100 mg B-1009 Brefeldin A 20350-15-6 >99% (TLC) 10 mg- $70 5 mg- $137 10 mg/ 100 mg B-1183 Bafilomycin A1 88899-55-2 >96% (HPLC) 1 mg- $176 10 μg- $176 1 mg B-1247 Blasticidin S HCl 3513-03-9 ≥98.0% 100 mg- $188 100 mg- $391 25 mg/ 100 mg B-2547 Bestatin 58970-76-6 >98% by HPLC 5 mg - $130 N/A 5 mg / 25 mg C-1019 CHAPS, High Purity 75621-03-3 ≥98.0% (HPLC) 100 g - $350.00 50 g - $841.00 10 g/ 100 g/ 1 kg C-1027 Cytochalasin B 14930-96-2 >98% (HPLC) 10 mg- $210 10 mg- $361