Review of Biomanipulation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Norfolk Local Flood Risk Management Strategy
Appendix A Norfolk Local Flood Risk Management Strategy Consultation Draft March 2015 1 Blank 2 Part One - Flooding and Flood Risk Management Contents PART ONE – FLOODING AND FLOOD RISK MANAGEMENT ..................... 5 1. Introduction ..................................................................................... 5 2 What Is Flooding? ........................................................................... 8 3. What is Flood Risk? ...................................................................... 10 4. What are the sources of flooding? ................................................ 13 5. Sources of Local Flood Risk ......................................................... 14 6. Sources of Strategic Flood Risk .................................................... 17 7. Flood Risk Management ............................................................... 19 8. Flood Risk Management Authorities ............................................. 22 PART TWO – FLOOD RISK IN NORFOLK .................................................. 30 9. Flood Risk in Norfolk ..................................................................... 30 Flood Risk in Your Area ................................................................ 39 10. Broadland District .......................................................................... 39 11. Breckland District .......................................................................... 45 12. Great Yarmouth Borough .............................................................. 51 13. Borough of King’s -

Efficacy of Using Electrofishing to Reduce Recruitment of Invasive Common Carp in Malheur Lake, a Large Endorheic Marsh
U.S. Fish and Wildlife Service Efficacy of using electrofishing to reduce recruitment of invasive Common Carp in Malheur Lake, a large endorheic marsh Final Report May 2017 By William Simpson, Doug Peterson , and Kurt Steinke Abernathy Fish Technology Center Longview, Washington Linda Beck1 Malheur Naonal Wildlife Refuge Princeton, Oregon 1 Current address: U.S. Fish and Wildlife Service, Fisheries and Aquac Conservaon, Portland, Oregon Adult Common Carp from Malheur Lake Developing embryos of Common Carp Abstract Concern about electrofishing inadvertently harming the embryos of species of conservation concern has motivated much of the research that describes what electrical conditions can kill fish embryos. As a result, targeted electrofishing might be underutilized as a potential control method to reduce the recruitment of nuisance or invasive fishes like Common Carp (Cyprinus carpio). Common Carp are one of the most widely distributed invasive fish in North America, and biologist are turning to integrated pest management techniques and targeting multiple life stages because control efforts based solely on removal of adults have often been unsuccessful. We examined changes in the survival of invasive Common Carp embryos while manipulating the transfer of electric power to the embryos by shocking them in water from carp-occupied areas using a variety of voltage gradient and waveform treatments produced by commercial electrofishing units and generators. Survival of electroshocked Common Carp embryos was < 50% at power densities (12,700 -

Georgian Farmhouse in Unspoilt Position
Georgian farmhouse in unspoilt position Grove House, Irstead, Norfolk Freehold Entrance hall • Drawing room • Dining room • Kitchen/ breakfast room with Aga • Study • Utility room • Old dairy Studio • Cloakroom/WC • Cellar • Six bedrooms • Family bathroom • Shower room • Outbuildings including barn Garaging • Mooring rights nearby • Mature gardens and grounds in all about 0.48 of an acre The Property traditionally constructed of red Grove House is a most brick and providing garaging attractive Grade II listed and workshop/storage space. Georgian farmhouse of circa The land in all extends to about 1820 with earlier origins 0.48 of an acre. believed to date to the 17th century. The house has elegant Situation and well-proportioned rooms Irstead is a small unspoilt rural typical of the period and lit by hamlet lying between Horning fine sash windows. Much and Neatishead. The village of period detail remains Neatishead is about half a mile throughout the house which and has a community village was acquired by the current shop and traditional pub. There owners in 1991 and who carried is a new village hall which hosts out a sensitive and faithful a number of local events. The programme of renovation and village of Irstead itself is about restoration. a mile and there is access via a boardwalk with fine walks Outside around the periphery of Barton The house is approached by a Broad nearby. The bustling gravelled drive to the east of riverside village of Horning the house which finishes in a (about two miles) has further large gravelled turning and everyday shopping including a parking space to the north of delicatessen, three public the house. -

Habitats Regulations Assessment of the South Norfolk Village Cluster Housing Allocations Plan
Habitats Regulations Assessment of the South Norfolk Village Cluster Housing Allocations Plan Regulation 18 HRA Report May 2021 Habitats Regulations Assessment of the South Norfolk Village Cluster Housing Allocations Plan Regulation 18 HRA Report LC- 654 Document Control Box Client South Norfolk Council Habitats Regulations Assessment Report Title Regulation 18 – HRA Report Status FINAL Filename LC-654_South Norfolk_Regulation 18_HRA Report_8_140521SC.docx Date May 2021 Author SC Reviewed ND Approved ND Photo: Female broad bodied chaser by Shutterstock Regulation 18 – HRA Report May 2021 LC-654_South Norfolk_Regulation 18_HRA Report_8_140521SC.docx Contents 1 Introduction ...................................................................................................................................................... 1 1.2 Purpose of this report ............................................................................................................................................... 1 2 The South Norfolk Village Cluster Housing Allocations Plan ................................................................... 3 2.1 Greater Norwich Local Plan .................................................................................................................................... 3 2.2 South Norfolk Village Cluster Housing Allocations Plan ................................................................................ 3 2.3 Village Clusters .......................................................................................................................................................... -

Genes to Ecosystems: Exploring the Frontiers of Ecology with One of the Smallest Biological Units
New Phytologist Review Tansley review Genes to ecosystems: exploring the frontiers of ecology with one of the smallest biological units 1 2 2 Author for correspondence: Adam S. Wymore , Annika T. H. Keeley , Kasey M. Yturralde , Thomas G. Whitham Melanie L. Schroer1, Catherine R. Propper1 and Thomas G. Whitham1,3 Tel: +1 928 523 7215 1 2 Email: [email protected] Department of Biological Sciences, Northern Arizona University, Flagstaff, AZ 86011, USA; School of Forestry, Northern Arizona University, Flagstaff, AZ 86011, USA; 3Merriam-Powell Center for Received: 9 December 2010 Accepted: 3 March 2011 Environmental Research, Northern Arizona University, Flagstaff, AZ 86011, USA Contents Summary 19 VI. Gene expression, fish and pollution 28 I. Introduction 19 VII. An emphasis on foundation species and 31 their biotic and abiotic interactions II. Fundamental principles and the community genetics 20 equivalent of Koch’s postulates VIII. Applications to the human condition 32 III. Genes, invasions and competition 21 IX. Conclusions 32 IV. Mutation, resistance and ecosystem consequences 24 Acknowledgements 33 V. Heritable traits, pine cones and climate 26 References 33 Summary New Phytologist (2011) 191: 19–36 Genes and their expression levels in individual species can structure whole commu- doi: 10.1111/j.1469-8137.2011.03730.x nities and affect ecosystem processes. Although much has been written about community and ecosystem phenotypes with a few model systems, such as poplar Key words: climate change, community and goldenrod, here we explore the potential application of a community genetics genetics postulates, ecosystem services, approach with systems involving invasive species, climate change and pollution. -

The Norfolk & Norwich
BRITISH MUSEUM (NATURAL HISTORY) TRANSACTIONS 2 7 JUN 1984 exchanged OF GENfcriAL LIBRARY THE NORFOLK & NORWICH NATURALISTS’ SOCIETY Edited by: P. W. Lambley Vol. 26 Part 5 MAY 1984 TRANSACTIONS OF THE NORFOLK AND NORWICH NATURALISTS’ SOCIETY Volume 26 Part 5 (May 1984) Editor P. W. Lambley ISSN 0375 7226 U: ' A M «SEUV OFFICERS OF THE SOCIETY 1984-85 j> URAL isSTORY) 2? JUH1984 President: Dr. R. E. Baker Vice-Presidents: P. R. Banham, A. Bull, K. B. Clarke, E. T. Daniels, K. C. Durrant, E. A. Ellis, R. Jones, M. J. Seago, J. A. Steers, E. L. Swann, F. J. Taylor-Page Chairman: Dr. G. D. Watts, Barn Meadow, Frost’s Lane, Gt. Moulton. Secretary: Dr. R. E. Baker, 25 Southern Reach, Mulbarton, NR 14 8BU. Tel. Mulbarton 70609 Assistant Secretary: R. N. Flowers, Heatherlands, The Street, Brundall. Treasurer: D. A. Dorling, St. Edmundsbury, 6 New Road, Heathersett. Tel. Norwich 810318 Assistant Treasurer: M. Wolner Membership Committee: R. Hancy, Tel. Norwich 860042 Miss J. Wakefield, Post Office Lane, Saxthorpe, NR1 1 7BL. Programme Committee: A. Bull, Tel. Norwich 880278 Mrs. J. Robinson, Tel. Mulbarton 70576 Publications Committee: R. Jones. P. W. Lambley & M. J. Seago (Editors) Research Committee: Dr. A. Davy, School of Biology, U.E.A., Mrs. A. Brewster Hon. Auditor. J. E. Timbers, The Nook, Barford Council: Retiring 1985; D. Fagg, J. Goldsmith, Miss F. Musters, R. Smith. Retiring 1986 Miss R. Carpenter, C. Dack, Mrs. J. Geeson, R. Robinson. Retiring 1987 N. S. Carmichael, R. Evans, Mrs.L. Evans, C. Neale Co-opted members: Dr. -

Habitats Regulations Assessment: Draft Scoping Report
North Norfolk 2016 - 2036 HABITATS REGULATIONS ASSESSMENT Draft Scoping Report May 2017 North Norfolk District Council Planning Policy Team Telephone: 01263 516318 E-Mail: [email protected] Write to: Planning Policy Manager, North Norfolk District Council, Holt Road, Cromer, NR27 9EN www.north-norfolk.gov.uk/localplan All documents can be made available in Braille, audio, large print or in other languages. Please contact 01263 516318 to discuss your requirements. Draft HRA Scoping - North Norfolk District Council – Emerging Local Plan Executive Summary Habitats Regulations Assessment is required in accordance with the Conservation of Habitats and Species Regulations 2010, (amended 2012); in order to ensure that plans and projects do not have a likely significant effect on any European designated sites for nature conservation. Such plans or projects can only proceed if the competent authority is convinced they will not have an “adverse effect on the integrity of a European site”. Where there is uncertainty over the effects then the competent authority will need to demonstrate how these can be avoided and what mitigation can be put in place. A Local Plan is the subject of Habitats Regulations Assessment, which is the responsibility of the plan-making body (in this case North Norfolk District Council) to produce. This scoping report provides the background and review of evidence to support the commencement of screening and the final Habitats Regulations Assessment of the North Norfolk Core Strategy and Site Allocations documents. This report considers: • The European designated sites within and outside the plan area affected. • The characteristics of these sites and their conservation objectives. -

The Addition of Artificial Macrophytes to Provide Macroinvertebrate Refugia at Alderfen Broad, Norfolk, England
Conservation Evidence (2006) 3, 58-60 www.ConservationEvidence.com The addition of artificial macrophytes to provide macroinvertebrate refugia at Alderfen Broad, Norfolk, England Hoare D., Jackson M.J. & Perrow M. The Broads Authority, 18 Colegate, Norwich, Norfolk NR3 1BQ, UK SUMMARY Nylon Italian cobweb brushes were added to Alderfen Broad to provide a refuge for zooplankton and other invertebrates. These were colonised quickly, initially largely by chironomids but then by a greater diversity of taxa. In the second year, there was a vigorous build-up of sponges. BACKGROUND further inputs of catchment-derived nutrients from entering the broad. Also, in 1992 a The Broads is an area of man-made, suction dredging operation was undertaken to interlinked shallow freshwater lakes in Norfolk remove nutrient rich mud from the bed of and Suffolk, eastern England. There are over Alderfen Broad to reduce phosphorus release 200 km of navigable waterways, with many (which encourages algal growth). more connecting small waterways such as streams and dykes. These link a variety of The purpose of artificial macrophytes: The habitats that support a rich diversity of flora Broads Authority, along with partners (English and fauna, including some of the rarest wildlife Nature and the Norfolk Wildlife Trust), in Britain. The main habitats are the rivers and initiated a project in 2000 to further restore broads (shallow lakes) themselves, fen, carr Alderfen Broad. Nutrient overload (nitrogen woodland and grazing marshes. Much of the and phosphorus) had lead to excessive growth water in the Broads has been affected by of algae and occasional 'blooms' of blue-green excessively high levels of phosphates and algae. -

The Eastern Counties, — ——
^^^^^ gh Guides : ——- h^ ==h* - c\J : :ct> r ^c\i ==^JQO - T— ""> h»- [~^co '-_ 7 —^^— :n UOUNTIES /t\u* ton ^¥/ua( vY "IP Grantham ' TaUdngh oihv Mort.ml l y'iii.oco..^i>s ^u , ! v , ^i,,:;;^ , i / v '"'''.v/,,. ;r~ nsiimV *\ ?. ' kXOton /lEICESTERY Monftw /{, r fontf* k ^> h'i .;-"" A0% .-O Krlmarsh\ Blisw.wfli.i2 'oad&J Eelmdon. "VTolvei J''u/<}, upthill r9tc Ami? LoAviibo- 'Widfc *Baldock effbhurn f J Marti}*?' Ihxatingfard eitfktoii 7 " gifzzarcL t^r ' t>un.sti ^OXFORD '/'> Ainershain. finest WytHtrnd^iL Bickuuuis>^ Watliagtnti >^Hi^TV^cHnb£ ^M Shxplake- jfe-wrffa^eR E A PI Nla ^ | J. Bartholomew", E3ix k 4t> fcs J«<00®»»®00 o ocoo iO>l>Ot>l>N0500 o o t-o •0000500^000 OOO o ft ,'rH0D»O0006Q0CMlO>LO H00«3 . o CD Ocp CO COO O O OOCOO ^•OOOOOOOOO o o o o Q 5 m taWOWOOOCO>OiO •io»oo>o CO rHrHrHrHi-HrHrHrHrH . rH rH rH rH ^•COOOOOOOOO _CO O O 3 ojlOrHOrHrHrHGOOO :* :'i>ho 3 rHrH<MrHrHt-lr-l<M<M . • rH rH <M O ft . ocococococoococo CO CO CO CO 3 • t» d- t~ i>- rH (MH^HHHIMiMN • <M <M rH <M •oooomooojohoiooo ^5 rH oJcO<NO<M^<MCOOOOOOCO<MO rHrHCQrHr-1 rHrHrH<MrH(MrHrH<M IrHOCOOOOOCOCOCO 00 O CO 'oo r3 :C5000^ocooooocooo o o Q 525 : oq : : : :§? : : : : : O a OQ r-4 : o • : : :^3 : : : : * a a o 3 O : : : : : : : : : : « : a ^ ft .ft .o • n • o3 • o •J25 o9 S • 0) cS . CO . :oq • :,3 : B :ra : flo -»j cS rQ 2 s.d tJD ? B fcr - 00 O ?+3 J* ^b-3 a p 5 3 8.5 g^ - » * +•+* * * H—H— -r-+-»-+-f-+* * +-+ * * -f--r- Tast. -

Restoration of the Littoral Margin by Removing Trees from the Lake Edge at Cockshoot Broad, Norfolk, England
Conservation Evidence (2006) 3, 71-72 www.ConservationEvidence.com Restoration of the littoral margin by removing trees from the lake edge at Cockshoot Broad, Norfolk, England Kelly A. & Southwood R.R. The Broads Authority, 18 Colegate, Norwich, Norfolk NR3 1BQ, UK SUMMARY Removal of overhanging alder Alnus glutinosa and grey sallow Salix cinerea trees from the edge of an East Anglian broad led to a vigorous growth of riparian plants around the water’s edge. BACKGROUND woodland and fen (Fig. 1). It is one of several broads where there is no access for boats at The Broads of Norfolk and Suffolk in eastern any time of the year, although they can moor at England, are Britain’s largest protected its entrance. A boardwalk is provided and wetland. They consist of 63 man-made, maintained by the Broads Authority to allow interlinked shallow freshwater lakes formed public access to the site. Despite being 600 years ago when medieval peat diggings declared as part of Bure Marshes National were flooded as water levels rose. There are Nature Reserve in 1958, Cockshoot Broad was over 200 km of navigable waterways, with severely affected by a build up of silt, resulting many more small watercourses, such as in turbid water, unsuitable for native flora and streams and dykes. These link a variety of fauna. habitats which support a rich diversity of wildlife, including some of the rarest flora and In 1982, Cockshoot Broad was isolated from fauna in Britain. the River Bure in an attempt to reduce the ingress of river water which, during that period In the Broads, the presence of a well developed contained an unacceptably high phosphorous littoral lake margin and a high proportion of level. -
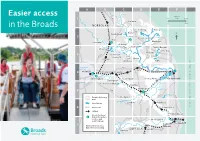
Easier Access Guide
A B C D E F R Ant Easier access A149 approx. 1 0 scale 4.3m R Bure Stalham 0 7km in the Broads NORFOLK A149 Hickling Horsey Barton Neatishead How Hill 2 Potter Heigham R Thurne Hoveton Horstead Martham Horning A1 062 Ludham Trinity Broads Wroxham Ormesby Rollesby 3 Cockshoot A1151 Ranworth Salhouse South Upton Walsham Filby R Wensum A47 R Bure Acle A47 4 Norwich Postwick Brundall R Yare Breydon Whitlingham Buckenham Berney Arms Water Gt Yarmouth Surlingham Rockland St Mary Cantley R Yare A146 Reedham 5 R Waveney A143 A12 Broads Authority Chedgrave area river/broad R Chet Loddon Haddiscoe 6 main road Somerleyton railway A143 Oulton Broad Broads National Park information centres and Worlingham yacht stations R Waveney Carlton Lowesto 7 Grid references (e.g. Marshes C2) refer to this map SUFFOLK Beccles Bungay A146 Welcome to People to help you Public transport the Broads National Park Broads Authority Buses Yare House, 62-64 Thorpe Road For all bus services in the Broads contact There’s something magical about water and Norwich NR1 1RY traveline 0871 200 2233 access is getting easier, with boats to suit 01603 610734 www.travelinesoutheast.org.uk all tastes, whether you want to sit back and www.broads-authority.gov.uk enjoy the ride or have a go yourself. www.VisitTheBroads.co.uk Trains If you prefer ‘dry’ land, easy access paths and From Norwich the Bittern Line goes north Broads National Park information centres boardwalks, many of which are on nature through Wroxham and the Wherry Lines go reserves, are often the best way to explore • Whitlingham Visitor Centre east to Great Yarmouth and Lowestoft. -

The Norfolk & Norwich
L c£H£RAl * 3 may ,*,UBRARY^ x TRANSACTIONS OF THE NORFOLK & NORWICH NATURALISTS’ SOCIETY Vol. 24 PART 4 APRIL 1978 ISBN 0375 7226 : : OFFICERS OF THE SOCIETY 1977-78 President: Ms D. M. Maxey ‘Greenfields’, Swanton Road, Dereham. President-Elect Mr P. J. Trett Victoria Road, Great Yarmouth Vice-Presidents: P. R. Banham, A. Bull, K. B. Clarke, E. T. Daniels, K. C. Durrant, E. A. Ellis, R. Jones, M. J. Seago, J. A. Steers, E. L. Swann, F. J. Taylor-Page General Secretary: R. E. Baker 25 Southern Reach, Mulbarton, NR14 8BU Tel. Mulbarton 70609 Assistant Secretary: (Membership and Publications) Ms J. Wakefield Post Office Lane, Saxthorpe, NR 11 7BL Assistant Secretary: (Minutes) P. W. Lambley Castle Museum, Norwich Excursion Secretary: Ms J. Robinson 5 Southern Reach, Mulbarton NR14 8BU Tel. Mulbarton 70576 Treasurer: D. A. Dorling St. Edmundsbury, 6 New Road, Hethersett Tel. Norwich 810318 Assistant Secretary: J. E. Timbers The Nook, Barford, Norfolk Editor: E. A. Ellis Wheatfen Broad, Surlingham, Norwich Auditor: E. L. Swann 282 Wootton Road, King’s Lynn, Norfolk Committee: M. J. Baker, G. Dunmore, Dr A. Davy (University Representative) Dr S. Cole, Ms C. Gurney, G. Hart, R. Hancy, P. W. Lambley (Museum Representative), J. Secker, Ms J. Smith, P. M. C. Stevens (Norfolk Naturalists’ Trust), P. Wright (Nature Conservancy Representative). ORGANISERS OF PRINCIPAL SPECIALIST GROUPS Birds {Editor of the Report) M. J. Seago, 33 Accacia Road, Thorpe, Norwich Mammals (Editor of the Report) R. Hancy, 124 Fakenham Road, Taverham NR8 6QH Plants: P. W. Lambley and E. L. Swann Fungi: E.