The Hypotensive and Uricosuric Effect of Valsartan Compared to Losartan in Gout Patients
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

207988Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 207988Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology Office of Medication Error Prevention and Risk Management RISK EVALUATION AND MITIGATION STRATEGY (REMS) REVIEW Date: December 20, 2015 Reviewer(s): Jasminder Kumar, PharmD Division of Risk Management (DRISK) Acting Team Leader: Jamie Wilkins Parker, Pharm.D., DRISK Deputy Director: Reema Mehta, Pharm.D., M.P.H., DRISK Director: Cynthia LaCivita, Pharm.D. Subject: Review evaluates if a REMS is needed for Zurampic (lesinurad) Drug Name: Zurampic (lesinurad) Therapeutic Class: Uricosuric agent Dosage form and route: 200mg tablet for oral administration Application Type/Number: NDA 207988 Applicant/Sponsor: Ardea Biosciences Inc. OSE RCM #: 2015-52 *** This document contains proprietary and confidential information that should not be released to the public. *** 1 Reference ID: 3863300 CONTENTS 1 INTRODUCTION ................................................................................................................... 3 1.1 Product Background ......................................................................................................... 3 1.2 Disease Background ......................................................................................................... 3 1.3 Regulatory History .......................................................................................................... -

Guidelines for Management of Gout. Part 1: Systematic Nonpharmacologic and Pharmacologic Therapeutic Approaches to Hyperuricemia DINESH KHANNA,1 JOHN D
Arthritis Care & Research Vol. 64, No. 10, October 2012, pp 1431–1446 DOI 10.1002/acr.21772 © 2012, American College of Rheumatology SPECIAL ARTICLE 2012 American College of Rheumatology Guidelines for Management of Gout. Part 1: Systematic Nonpharmacologic and Pharmacologic Therapeutic Approaches to Hyperuricemia DINESH KHANNA,1 JOHN D. FITZGERALD,2 PUJA P. KHANNA,1 SANGMEE BAE,2 MANJIT K. SINGH,3 TUHINA NEOGI,4 MICHAEL H. PILLINGER,5 JOAN MERILL,6 SUSAN LEE,7 SHRADDHA PRAKASH,2 MARIAN KALDAS,2 MANEESH GOGIA,2 FERNANDO PEREZ-RUIZ,8 WILL TAYLOR,9 FRE´ DE´ RIC LIOTE´ ,10 HYON CHOI,4 JASVINDER A. SINGH,11 NICOLA DALBETH,12 SANFORD KAPLAN,13 VANDANA NIYYAR,14 DANIELLE JONES,14 STEVEN A. YAROWS,15 BLAKE ROESSLER,1 GAIL KERR,16 CHARLES KING,17 GERALD LEVY,18 DANIEL E. FURST,2 N. LAWRENCE EDWARDS,19 BRIAN MANDELL,20 H. RALPH SCHUMACHER,21 MARK ROBBINS,22 2 7 NEIL WENGER, AND ROBERT TERKELTAUB Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to these guidelines and recommendations to be voluntary, with the ultimate determi- nation regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed or endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice. -

Lesinurad Cuts Uric Acid in Refractory Gout
20 ARTHRITIS AUGUST 2011 • RHEUMATOLOGY NEWS Lesinurad Cuts Uric Acid in Refractory Gout Ninety percent of patients who remained on fore the end of this year, said Barry D. a uric acid transporter molecule in the Quart, Pharm.D., president of Ardea kidney that takes uric acid out of urine treatment for 28 weeks met the study’s target level. Biosciences, the San Diego–based com- and places it back into the blood. pany that is developing the drug. The study led by Dr. Perez-Ruiz en- BY MITCHEL L. ZOLER mg/day of lesinurad also appeared safe, The phase III study will likely focus on rolled patients who met the 1977 gout di- with no adverse effects or other safety the 200-mg and 400-mg/day dosages, as agnosis criteria of the American FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY concerns seen during the limited treat- over time most patients appeared to re- Rheumatism Association (now the ment period reported, said Dr. Perez- spond to these lower dosages. American College of Rheumatology) LONDON – An investigational drug Ruiz, a rheumatologist at Hospital de “Almost no one needs 600 mg/day to and who had a serum uric acid level that boosts uric acid secretion led to sig- Cruces in Barakaldo, Spain. get a response,” Dr. Quart said in an in- greater than 6 mg/dL despite being on terview. a stable dose of 200-600 mg allopurinol Major Finding: After 4 weeks of treatment with a combination of 200-600 Although the current study used pa- for at least 6 weeks. -

COZAAR (Losartan Potassium) Tablets, for Oral Use
HIGHLIGHTS OF PRESCRIBING INFORMATION • Increase dose to 100 mg once daily if further blood pressure These highlights do not include all the information needed to use response is needed. (2.3) COZAAR safely and effectively. See full prescribing information for COZAAR. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 25 mg; 50 mg; and 100 mg. (3) ® COZAAR (losartan potassium) tablets, for oral use ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 1995 • Hypersensitivity to any component. (4) • Coadministration with aliskiren in patients with diabetes. (4) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Hypotension: Correct volume or salt depletion prior to administration When pregnancy is detected, discontinue COZAAR as soon as of COZAAR. (5.2) possible. Drugs that act directly on the renin-angiotensin system • Monitor renal function and potassium in susceptible patients. (5.3, can cause injury and death to the developing fetus. (5.1) 5.4) --------------------------- RECENT MAJOR CHANGES --------------------------- ------------------------------ ADVERSE REACTIONS ------------------------------ Warnings and Precautions Hyperkalemia (5.4) 10/2018 Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, ----------------------------INDICATIONS AND USAGE ---------------------------- and back pain. (6.1) COZAAR is an angiotensin II receptor blocker (ARB) indicated for: • Treatment of hypertension, to lower blood pressure in adults and To report SUSPECTED ADVERSE REACTIONS, contact Merck children greater than 6 years old. Lowering blood pressure reduces Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877- the risk of fatal and nonfatal cardiovascular events, primarily strokes 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28
COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28 COZAAR® (LOSARTAN POTASSIUM TABLETS) USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, COZAAR® should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality. DESCRIPTION COZAAR (losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non- peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole- 5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is: Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan. COZAAR is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium and the following inactive ingredients: microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. COZAAR 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively. COZAAR 25 mg, COZAAR 50 mg, and COZAAR 100 mg may also contain carnauba wax. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Telmisartan Vs Losartan Plus Hydrochlorothiazide in the Treatment of Mild-To-Moderate Essential Hypertensionfa Randomised ABPM Study
Journal of Human Hypertension (2003) 17, 569–575 & 2003 Nature Publishing Group All rights reserved 0950-9240/03 $25.00 www.nature.com/jhh ORIGINAL ARTICLE Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertensionFa randomised ABPM study JM Neutel1 , RE Kolloch2, PF Plouin3, TW Meinicke4 and H Schumacher5 for the OTELLOH Study Group 1Orange County Heart Institute & Research Center, Orange, CA, USA; 2Krankenanstalten Gilead, Bielefeld, Germany; 3Hoˆpital Broussais, Paris, France; 4Clinical Research Boehringer Ingelheim Pharma KG, Biberach, Germany; 5Medical Data Services Boehringer Ingelheim Pharma KG, Ingelheim, Germany The objective of this prospective, randomised, open- null hypothesis of a treatment difference of 43 mmHg. label, blinded-end point parallel-group, multicentre The reduction in mean 24-h systolic blood pressure was study was to show that telmisartan 80 mg is not inferior 13.2 7 10.2 mmHg with telmisartan and 17.1 7 10.3 to a fixed-dose combination of losartan 50 mg/hydro- mmHg with losartan/HCTZ. Both drugs provided effec- chlorothiazide (HCTZ) 12.5 mg in patients with mild-to- tive control over the 24-h dosing interval. Analyses of moderate hypertension. The criterion for noninferiority morning (0600–1159) ambulatory blood pressure mon- was a treatment difference of p3.0 mmHg in the reduc- itoring DBP means and trough cuff DBP confirmed the tion of 24-h mean ambulatory diastolic blood pressure noninferiority hypothesis of the protocol for telmisartan (DBP) from the end of the 4-week placebo washout 80 mg vs losartan 50 mg/HCTZ 12.5 mg. The reductions period to the end of the 6-week active treatment period. -
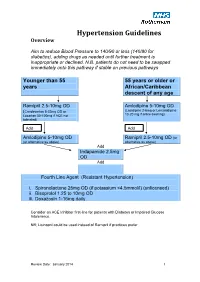
Hypertension Protocol
Hypertension Guidelines Overview Aim to reduce Blood Pressure to 140/90 or less (140/80 for diabetics), adding drugs as needed until further treatment is inappropriate or declined. N.B. patients do not need to be swapped immediately onto this pathway if stable on previous pathways Younger than 55 55 years or older or years African/Caribbean descent of any age Ramipril 2.5-10mg OD Amlodipine 5-10mg OD (Candesartan 8-32mg OD or (Lacidipine 2-6mg or Lercanidipine 10-20 mg if ankle swelling) Losartan 50-100mg if ACE not tolerated) Add Add Amlodipine 5-10mg OD Ramipril 2.5-10mg OD (or (or alternative as above) alternative as above) Add Indapamide 2.5mg OD Add Fourth Line Agent (Resistant Hypertension) i. Spironolactone 25mg OD (if potassium <4.5mmol/l) (unlicensed) ii. Bisoprolol 1.25 to 10mg OD iii. Doxazosin 1-16mg daily Consider an ACE Inhibitor first-line for patients with Diabetes or Impaired Glucose Intolerance. NB; Lisinopril could be used instead of Ramipril if practices prefer Review Date: January 2014 1 Review Date: January 2014 2 General Principles 1. Clinic BP >140/90 leads to Ambulatory Blood Pressure Monitoring (ABPM) 2. ABPM uses average of at least 14 readings taken during waking hours 3. Offer antihypertensive drug to all under 80 with Stage 1 hypertension with: o Target organ damage (high albumin:creatinine ratio, haematuria, raised creatinine, low eGFR, hypertensive retinopathy, LVH) o Established cardiovascular disease o Renal disease o Diabetes o 10 year Cardiovascular risk >20% 4. Offer antihypertensive drug to all with stage 2 hypertension 5. -

AT1-Receptor Blockers: Differences That Matter
Journal of Human Hypertension (2002) 16, S9–S16 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh AT1-receptor blockers: differences that matter AH Gradman Division of Cardiovascular Diseases, The Western Pennsylvania Hospital, Pittsburgh, Pennsylvania, USA The available angiotensin II type 1 (AT1)-receptor block- effect, and this finding is supported by the results of ers differ markedly in their pharmacological properties comparative clinical trials. Moreover, the prolonged and clinical efficacy. Losartan shifts the dose-response binding of candesartan to the receptor is reflected in a curve for angiotensin II to the right without affecting the longer duration of action, compared with losartan; the maximal response; this antagonism can be overcome by antihypertensive effect of candesartan persists for 48 h increasing concentrations of angiotensin II and thus los- after dosing, compared with approximately 24 h with artan acts as a surmountable antagonist. By contrast, losartan. Candesartan thus offers extended therapeutic other agents suppress the maximal response to angio- coverage, an important consideration since a majority tensin II to varying extents; this can not be overcome by of patients miss occasional doses of antihypertensive increasing angiotensin concentrations and hence these medication. There is currently no evidence that differ- agents are insurmountable antagonists. Receptor bind- ences in receptor binding between AT1-receptor block- ing studies have shown that candesartan has the high- ers translate into differences in tolerability. In summary, est affinity for the AT1-receptor, followed by irbesartan, therefore, pharmacological differences between AT1- valsartan and losartan, and that candesartan dis- receptor blockers are reflected in clinically important sociates from the receptor more slowly than other differences in maximal antihypertensive effect, antagonists. -

Therapeutic Class Overview Angiotensin II Receptor Blockers (Arbs) – Combination Products
Therapeutic Class Overview Angiotensin II receptor blockers (ARBs) – combination products Therapeutic Class • Overview/Summary: This review will focus on the angiotensin II receptor blocker (ARB) combination products.1-13 The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.14,15 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.16 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.14,16 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including hypertrophy and remodeling.14,15 The RAAS plays an important role in the development and progression of heart failure.15 ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator associated with dry cough.14-17 Since angiotensin II may also be generated through other pathways that do not depend upon ACE (e.g., chymase), blockade of angiotensin II by ACE inhibitors is incomplete.14,15 The ARBs block the angiotensin II receptor subtype AT1, preventing the negative effects of angiotensin II, regardless of its origin. ARBs do not appear to affect bradykinin. Amlodipine, a nondihydropyridine calcium channel blocker, inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Cardiac and vascular smooth muscle contraction depends on the movement of extracellular calcium ions into cells through specific ion channels. -

Therapeutic Class Overview Renin Inhibitors and Combinations
Therapeutic Class Overview Renin Inhibitors and Combinations INTRODUCTION Approximately 92.1 million American adults have at least one type of cardiovascular disease according to the 2017 American Heart Association Heart Disease and Stroke Statistics update. From 2004 to 2014, mortality associated with cardiovascular disease declined 25.3% (Benjamin et al, 2017). An estimated 85.7 million Americans or 34% of US adults aged ≥20 years have high blood pressure (BP). Hypertension is an independent risk factor for cardiovascular disease and increases the mortality risks of cardiovascular disease and other diseases (Benjamin et al, 2017). Lowering of BP has been shown to reduce the risk of fatal and nonfatal cardiovascular events including stroke and myocardial infarctions (MI) improving cardiovascular health and reducing cardiovascular risk also includes lipid control, diabetes management, smoking cessation, exercise, weight management, and limited sodium intake (Benjamin et al, 2017). Aliskiren (TEKTURNA®) is the only single entity direct renin inhibitor available in the United States (U.S.) and is Food and Drug Administration (FDA)-approved for the treatment of hypertension, either as monotherapy or in combination with other antihypertensive agents. Currently, only one combination renin inhibitor product is available in the US. This product combines the direct renin inhibitor, aliskiren, with a thiazide diuretic (TEKTURNA-HCT®) and is approved for hypertension. Studies have demonstrated that the combination of two inhibitors of the renin angiotensin system (RAS), including aliskiren, an angiotensin converting enzyme inhibitor (ACE-I) or an angiotensin II receptor blocker (ARB), provide no renal or cardiovascular benefits, and significant adverse events, particularly in patients with diabetes and/or renal insufficiency. -

Risk of Severe COVID-19 Disease with ACE Inhibitors and Angiotensin
Special populations ORIGINAL RESEARCH Heart: first published as 10.1136/heartjnl-2020-317393 on 31 July 2020. Downloaded from Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people Julia Hippisley- Cox ,1 Duncan Young,2,3 Carol Coupland,4 Keith M Channon,5 Pui San Tan,6 David A Harrison ,7 Kathryn Rowan,8 Paul Aveyard,6 Ian D Pavord,9 Peter J Watkinson 5,10 For numbered affiliations see ABSTRACT (COVID-19) in the UK were confirmed on 24 end of article. Background There is uncertainty about the January 2020. Since then the disease has spread associations of angiotensive enzyme (ACE) inhibitor and rapidly through the population. There are no Correspondence to angiotensin receptor blocker (ARB) drugs with COVID-19 vaccines, preventative or curative treatments for Prof Julia Hippisley- Cox, Primary Care Health Sciences, University disease. We studied whether patients prescribed these COVID-19 disease and only one possible disease- 1 of Oxford, Oxford OX1 2JD, UK; drugs had altered risks of contracting severe COVID-19 modifying treatment so the government has used Julia. Hippisley- Cox@ phc. ox. disease and receiving associated intensive care unit (ICU) social distancing as a population-level intervention ac. uk admission. to limit the rate of increase in cases. Methods This was a prospective cohort study using Case series of confirmed COVID-19 have iden- Received 20 May 2020 2 3 2 4 5 Revised 8 July 2020 routinely collected data from 1205 general practices in tified age, sex, comorbidities and ethnicity as Accepted 13 July 2020 England with 8.28 million participants aged 20–99 years.