'Pseudo'- Syndromes in Cardiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiology-EKG Michael Bradley
Cardiology/EKG Board Review Michael J. Bradley D.O. DME/Program Director Family Medicine Residency Objectives • Review general method for EKG interpretation • Review specific points of “data gathering” and “diagnoses” on EKG • Review treatment considerations • Review clinical cases/EKG’s • Board exam considerations EKG EKG – 12 Leads • Anterior Leads - V1, V2, V3, V4 • Inferior Leads – II, III, aVF • Left Lateral Leads – I, aVL, V5, V6 • Right Leads – aVR, V1 11 Step Method for Reading EKG’s • “Data Gathering” – steps 1-4 – 1. Standardization – make sure paper and paper speed is standardized – 2. Heart Rate – 3. Intervals – PR, QT, QRS width – 4. Axis – normal vs. deviation 11 Step Method for Reading EKG’s • “Diagnoses” – 5. Rhythm – 6. Atrioventricular (AV) Block Disturbances – 7. Bundle Branch Block or Hemiblock of – 8. Preexcitation Conduction – 9. Enlargement and Hypertrophy – 10. Coronary Artery Disease – 11. Utter Confusion • The Only EKG Book You’ll Ever Need Malcolm S. Thaler, MD Heart Rate • Regular Rhythms Heart Rate • Irregular Rhythms Intervals • Measure length of PR interval, QT interval, width of P wave, QRS complex QTc • QTc = QT interval corrected for heart rate – Uses Bazett’s Formula or Fridericia’s Formula • Long QT syndrome – inherited or acquired (>75 meds); torsades de ponites/VF; syncope, seizures, sudden death Axis Rhythm • 4 Questions – 1. Are normal P waves present? – 2. Are QRS complexes narrow or wide (≤ or ≥ 0.12)? – 3. What is relationship between P waves and QRS complexes? – 4. Is rhythm regular or irregular? -

Parasystole in a Mahaim Accessory Pathway
223 Case Report Parasystole in a Mahaim Accessory Pathway Chandramohan Ramasamy MD, Senthil Kumar MD, Raja J Selvaraj MD, DNB Department of Cardiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India Address for Correspondence: Dr. Raja J Selvaraj, Assistant Professor of Cardiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry - 605006, India. E-Mail: [email protected] Abstract Automaticity has been described in Mahaim pathways, both spontaneously and during radiofrequency ablation. We describe an unusual case of automatic rhythm from a Mahaim pathway presenting as parasystole. The parasystolic beats were also found to initiate tachycardia, resulting in initial presentation with incessant tachycardia and tachycardia induced cardiomyopathy. Key words: Mahaim tachycardia, Parasystole, Automaticity Introduction Mahaim pathways are atriofascicular accessory pathways with decremental, anterograde only conduction. The most common clinical manifestation related to these pathways is antidromic reentrant tachycardia. Less commonly, the pathway may be a bystander with atrioventricular nodal reentrant tachycardia or atrial tachycardia. Rarely, automaticity has been reported from the pathway, manifesting as ectopic beats during sinus rhythm or as an automatic tachycardia [1,2]. Parasystole is a condition where an ectopic focus is unaffected by the underlying rhythm due to entrance block. Parasystole has been reported from atrial musculature, ventricular musculature -

Brugada Syndrome Associated to Myocardial Ischemia Sindrome De Brugada Associado a Isquemia Miocárdica
Brugada syndrome associated to Myocardial ischemia Sindrome de Brugada associado a isquemia miocárdica Case of Dr Raimundo Barbosa Barros From Fortaleza - Ceará - Brazil Caro amigo Dr. Andrés Gostaria de ouvir a opinião dos colegas do foro sobre este paciente masculino 56anos internado na emergência do nosso hospital dia 03 de Novembro de 2010. Relata que em abril deste ano foi internado por quadro clínico compatível com angina instável (ECG1). Na ocasião foi submetido à coronariografia que revelou lesão crítica proximal da arteria descendente anterior e lesão de 90% na porção distal da artéria coronária direita. Nesta ocasião realizou angioplastia com colocação de stent apenas na artéria descendente anterior ( ECG2 pós ATC) A artéria coronaria direita não foi abordada. O paciente evoluiu assintomático(ECG3). Em 20/09/2010 realizou cintilografia miocárdica de rotina que resultou normal. No dia 03 de novembro de 2010 procura emergência refirindo ter sofrido episódio de sincope precedido de palpitações rápidas e desconforto torácico atípico.(ECGs 4 e 5). Adicionalmente, informa que 4 horas antes da sua admissão havia apresentado febre (não documentada). Não há relato de episódio prévio semelhante ou história familiar positiva para Morte súbita em familiar jovem de primeiro grau. Dosagem seriada de CK-MB e troponina normais. Qual os diagnósticos ECGs e qual a conduta? Um abraço para todos Raimundo Barbosa Barros Fortaleza Ceará Brasil Dear friend, Dr. Andrés, I would like to know the opinion from the colleagues of the forum about this patient (male, 56 years old), admitted in the ER of our hospital, on November 3rd, 2010. He claims that in April of this year he was admitted with symptoms of unstable angina (ECG1). -

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

Abnormal ECG Findings in Athletes Normal ECG
SEATTLE CRITERIA Abnormal ECG findings in athletes These ECG findings are unrelated to regular training or expected physiologic adaptation to exercise, may suggest the presence of pathologic cardiovascular disease, and require further diagnostic evaluation. Abnormal ECG finding Definition T wave inversion > 1 mm in depth in two or more leads V2-V6, II and aVF, or I and aVL (excludes III, aVR, and V1) ST segment depression ≥ 0.5 mm in depth in two or more leads Pathologic Q waves > 3 mm in depth or > 40 ms in duration in two or more leads (except III and aVR) Complete left bundle branch block QRS ≥ 120 ms, predominantly negative QRS complex in lead V1 (QS or rS), and upright monophasic R wave in leads I and V6 Intra-ventricular conduction delay Any QRS duration ≥ 140 ms Left axis deviation -30° to -90° Left atrial enlargement Prolonged P wave duration of > 120 ms in leads I or II with negative portion of the P wave ≥ 1 mm in depth and ≥ 40 ms in duration in lead V1 Right ventricular hypertrophy R-V1 + S-V5 > 10.5 mm and right axis deviation > 120° pattern Ventricular pre-excitation PR interval < 120 ms with a delta wave (slurred upstroke in the QRS complex) and wide QRS (> 120 ms) Long QT interval* QTc ≥ 470 ms (male) QTc ≥ 480 ms (female) QTc ≥ 500 ms (marked QT prolongation) Short QT interval* QTc ≤ 320 ms Brugada-like ECG pattern High take-off and downsloping ST segment elevation followed by a negative T wave in ≥ 2 leads in V1-V3 Profound sinus bradycardia < 30 BPM or sinus pauses ≥ 3 sec Mobitz type II 2° AV block Intermittently non-conducted P waves not preceded by PR prolongation and not followed by PR shortening 3° AV block Complete heart block Atrial tachyarrhythmias Supraventricular tachycardia, atrial fibrillation, atrial flutter Premature ventricular contractions ≥ 2 PVCs per 10 second tracing Ventricular arrhythmias Couplets, triplets, and non-sustained ventricular tachycardia *The QT interval corrected for heart rate is ideally measured with heart rates of 60-90 bpm. -

Electrocardiography in Aortic Regurgitation: It's in the Details
THE CLINICAL PICTURE SRIDHAR VENKATACHALAM, MD, MRCP CURTIS M. RIMMERMAN, MD, MBA Department of Hospital Medicine, Cleveland Clinic Gus P. Karos Chair, Clinical Cardiovascular Medicine, Department of Cardiovascular Medicine, Cleveland Clinic The Clinical Picture Electrocardiography in aortic regurgitation: It’s in the details Although not routinely reported, the negative U wave indicates underlying FIGURE 1. The patient’s 12-lead electrocardiogram shows normal sinus rhythm and a rate of 55 beats per minute. The frontal-plane QRS complex vector is deviated leftward. Left atrial abnor- structural or mality is present, given the terminally negative P wave in lead V1 and the bifid P wave in lead II. ischemic heart Left ventricular volume overload is supported by the following findings: increased QRS complex voltage, best seen in the precordial (chest) leads, indicative of increased left ventricular mass; disease prominent septal depolarization, as reflected by Q waves in leads 4V to V6; the absence of an ST- segment or T-wave abnormality; and negative U waves in leads V4 to V6 (arrows). 72-year-old man with a 15-year history taking any cardiac drugs, and his health has A of a heart murmur presents to his cardi- previously been excellent. ologist with shortness of breath on exertion On physical examination, his pulse rate is over the past 12 months. He otherwise feels regular at 55 beats per minute, and his blood well and reports no chest discomfort, palpita- pressure is 178/66 mm Hg. Cardiac ausculta- tions, or swelling of his legs or feet. He is not tion reveals an early- to mid-diastolic murmur heard best with the patient seated and leaning doi:10.3949/ccjm.78a.10141 forward, and located at the left sternal border; CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 78 • NUMBER 8 AUGUST 2011 505 Downloaded from www.ccjm.org on October 2, 2021. -

Morphological Abnormalities in Baseline Ecgs in Healthy Normal Volunteers Participating in Phase I Studies
Indian J Med Res 135, March 2012, pp 322-330 Morphological abnormalities in baseline ECGs in healthy normal volunteers participating in phase I studies Pooja Hingorani, Mili Natekar, Sheetal Deshmukh, Dilip R. Karnad, Snehal Kothari, Dhiraj Narula & Yash Lokhandwala Research Section, Quintiles Cardiac Safety Services, Mumbai, India Received September 30, 2010 Background & objectives: Morphological abnormalities in 12-lead electrocardiograms (ECGs) are seen in subgroups of healthy individuals like athletes and air-force personnel. As these populations may not truly represent healthy individuals, we assessed morphological abnormalities in ECG in healthy volunteers participating in phase I studies, who are screened to exclude associated conditions. Methods: ECGs from 62 phase I studies analyzed in a central ECG laboratory were pooled. A single drug-free baseline ECG from each subject was reviewed by experienced cardiologists. ECG intervals were measured on five consecutive beats and morphological abnormalities identified using standard guidelines. Results: Morphological abnormalities were detected in 25.5 per cent of 3978 healthy volunteers (2495 males, 1483 females; aged 18-76 yr); the presence was higher in males (29.3% vs. 19.2% in females; P<0.001). Rhythm abnormalities were the commonest (11.5%) followed by conduction abnormalities (5.9%), axis deviation (4%), ST-T wave changes (3.1%) and chamber enlargement (1.4%). Incomplete right bundle branch block (RBBB), short PR interval and right ventricular hypertrophy were common in young subjects (<20 yr) while atrial fibrillation, first degree atrioventricular block, complete RBBB and left anterior fascicular block were more prevalent in elderly subjects (>65 yr). Prolonged PR interval, RBBB and intraventricular conduction defects were more common in males while sinus tachycardia, short PR interval and non-specific T wave changes were more frequent in females. -

12 Lead Ekgs
12 Lead ECG Interpretation: Using A Systematic Approach (Part 2= Axis) Leslie L Davis, PhD, RN, ANP-BC, FAANP, FAHA UNC Greensboro, School of Nursing No disclosures relevant to this presentation. What is Meant by Axis? •Net direction of electrical vector during ventricular depolarization – Average direction of current flow – As the ventricles depolarize the direction of current flows leftward & downward b/c most of the ventricular mass is on the left Source: Author: Rob Kreuger Medical illustrator, AMC, The Netherlands avail at: http://en.ecgpedia.org/wiki/File:Hartas2.jpg Systematic Interpretation of 12 Lead EKGs –Step 2: Determine axis • Determined by looking at 6 frontal plane leads • Leads I & aVF most often used – Some sources use Leads I & II • Variable among individuals Courtesy of Dr. Nicholas Patchett. Available through creative commons via Wikipedia at: https://en.wikipedia.org/wiki/Electrocardiography#/media/File:EKG_leads.png Determining Axis The QRS axis is determined by overlying a circle, in the frontal plane. By convention, the degrees of the circle are as shown. The normal QRS axis lies between -10o and +110o. A QRS axis that falls between -10o and -90o is abnormal and called left axis deviation. A QRS axis that falls between +110o and +180o is abnormal and called right axis deviation. A QRS axis that falls between +180o and -90o is abnormal and called extreme right axis deviation. Courtesy of Dr. De Voogt & ECGpedia.org http://nl.ecgpedia.org/images/9/91/ECG_lead_angulation.png Thaler, 2007 Causes of Left Axis Deviation -

Left Bundle Branch Block with Intermittent QRS Axis Switching Observation of a Hypertensive Patient for 18 Years
Case Reports Left Bundle Branch Block With Intermittent QRS Axis Switching Observation of a Hypertensive Patient for 18 Years Tetsuya Takato,1 MD, Namie Yamada,1 MD, Jun Fujii,1 MD, Saburo Mashima,2 MD, and Terunao Ashida,1 MD Summary A 64-year-old man who had been prescribed antihypertensive drugs since 1971 at- tended our clinic in 1988 with hypertension and electrocardiographic abnormalities. An electrocardiogram revealed left axis deviation (LAD) in 1988 and slightly prolonged PQ intervals in 1993. Complete left bundle branch block (CLBBB) with LAD developed in May 1995. The wide QRS of the CLBBB had never returned to the normal narrow QRS and had intermittently alternated between LAD and normal axis. The PQ intervals were longer when the QRS axis showed LAD compared to that with normal QRS axis. The QRS complexes in leads V1-V3 revealed an R wave at LAD and a QS pattern at normal axis. During a deep breathing test, the QRS axis switched from normal axis to LAD at the end of forced expiration and also switched from normal axis to LAD within a few minutes after the exercise test. These results suggest that the shift of the QRS axis might be related to the tone of the autonomic nervous system. (Int Heart J 2009; 50: 677-684) Key words: Left bundle branch block, QRS axis alternation, Electrocardiogram LEFT bundle branch block (LBBB) usually occurs in patients with cardiac disease. However, Rosenbaum and others1-3) have reported that as many as 12% of patients with LBBB have no demonstrable heart disease. -
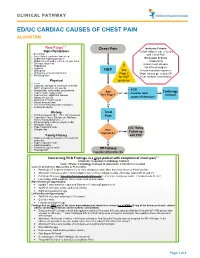
Ed/Uc Cardiac Causes of Chest Pain Algorithm
CLINICAL PATHWAY ED/UC CARDIAC CAUSES OF CHEST PAIN ALGORITHM 1,2 Red Flags Chest Pain Inclusion Criteria Signs/Symptoms Verbal children, age 2-22 yrs • Exertional with Chest Pain • Acute Onset, awakens from sleep • Substernal crushing pressure Exclusion Criteria • Radiation to shoulder, arm, neck, jaw, back Ill appearing • Syncope, dizziness Known heart disease • Palpitations • Dyspnea ! Hx of heart surgery • Orthopnea H&P See Known ingestion/exposure • Pulmonary embolus risk factors Page 2 Major trauma preceding CP • Illicit Drug Use for H&P Acute asthma exacerbation Physical guidance • Fever • Cyanosis, tachypnea, shortness of breath, WOB, abnormal breath sounds • • ECG Bradycardia, tachycardia, dysrhythmia Any Cardiology • Hypertension, hypotension Yes • Consider other • New murmur, significant murmur Red Flags? Consult • Gallop, friction rub causes of chest pain • Abnormal 2nd heart sound • Distant heart sounds • Decreased femoral/peripheral pulses No • Peripheral edema History Treat • Arthritis/vasculitis (SLE, IBD, JIA, Kawasaki) • Connective Tissue Disease (ie. Marfans, Pain Ehlers-Danlos Syndrome…) • QT-prolonging meds (ex. psych meds) • Oncologic history • Hypercoagulable state D/C Home • Dyslipidemia Pain Yes Follow up Improved? Family History with PCP • Sudden unexplained death or MI under 40 years old • Hypercoagulable state No • Cardiomyopathy • Pulmonary hypertension Off Pathway • Prolonged QT Consider Alternative Dx Concerning ECG Findings in a >2yo patient with complaint of chest pain1,2 Anschutz: In-person Cardiology -
What Are ECG Normal Variants?
Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 1 of 6 other concerns. Let’s look at a few examples of ECG Normal Variants... Sinus Bradycardia Sinus bradycardia is a normal variant if the pilot is 49 or younger, and their heart rate is greater than 44 beats per minute. At age 50 and older, their heart rate must be greater than 48 beats per minute. Sinus Tachycardia Sinus tachycardia is a normal variant if the pilot’s heart rate is less than 110 beats per minute. Sinus Arrhythmia Sinus arrhythmia is a normal variant. Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 2 of 6 other concerns. Let’s look at a few examples of ECG Normal Variants... Low Atrial Rhythm Low atrial rhythm is a normal variant when there are upright P waves in the AVR lead, and inverted P’s in other limb leads with a short PR interval. Ectopic Atrial Rhythm With ectopic atrial rhythm, the S.A. node rhythm originates in multiple areas of the atria. Wandering Atrial Pacemaker A wandering atrial pacemaker looks similar to ectopic atrial rhythm, depending on the interpretive output of your diagnostic equipment. Many common ECG findings What are ECG are normal variants and are not cause for deferment, unless the pilot is Normal Variants? symptomatic or there are page 3 of 6 other concerns. -

ECG Conduction Delays: a Case of Viral Myocarditis
ECG conduction delays: A case of viral myocarditis Elizabeth Dobben, Sherene Lattimore and Julie L. Welch 23 yo F presented to the ED with 5 days of malaise, abdominal pain, vomiting, and dyspnea. Physical examination revealed tachycardia and right upper quadrant tenderness. IV fluids and symptom control were started. Her basic laboratory results were unremarkable. An Abdominal CT with IV contrast showed periportal edema and a small pericardial effusion. An ECG revealed atrial tachycardia with 2:1 conduction and a right bundle branch block. She was placed on a cardiac monitor and Cardiology was consulted. Serial ECG's revealed a widening QRS with conduction delays and eventual complete heart block on the monitor. An ECHO showed global hypokinesis with an EF of 34%. Troponin and BNP were elevated. She underwent an emergent cardiac catheterization and pacemaker placement. In the ICU she decompensated, requiring ECMO support for 2 weeks. After a prolong hospital stay, she was discharged with and EF of 66% and a diagnosis of Coxsackie B viral myocarditis with cardiomyopathy. ECG #1 (08:02 am): Ventricular rate 64 bpm. Atrial tachycardia rate 128 bpm with 2:1 AV conduction. Left axis deviation. Right bundle branch block with QRS 124 ms. Left anterior fascicular block. QTc 439. ECG #2 (09:02 am): Ventricular rate 60 bpm. Atrial tachycardia rate 133 bpm with variable AV conduction, concerning for developing third-degree heart block. Nonspecific intraventricular block (QRS 154 ms) with alternating right and left bundle branch blocks, suggesting advance conduction system disease. QTc 502. ECG #3 (16:19 pm after cardiac catheterization and pacemaker placement): Ventricular-paced rhythm with rate 80 bpm.