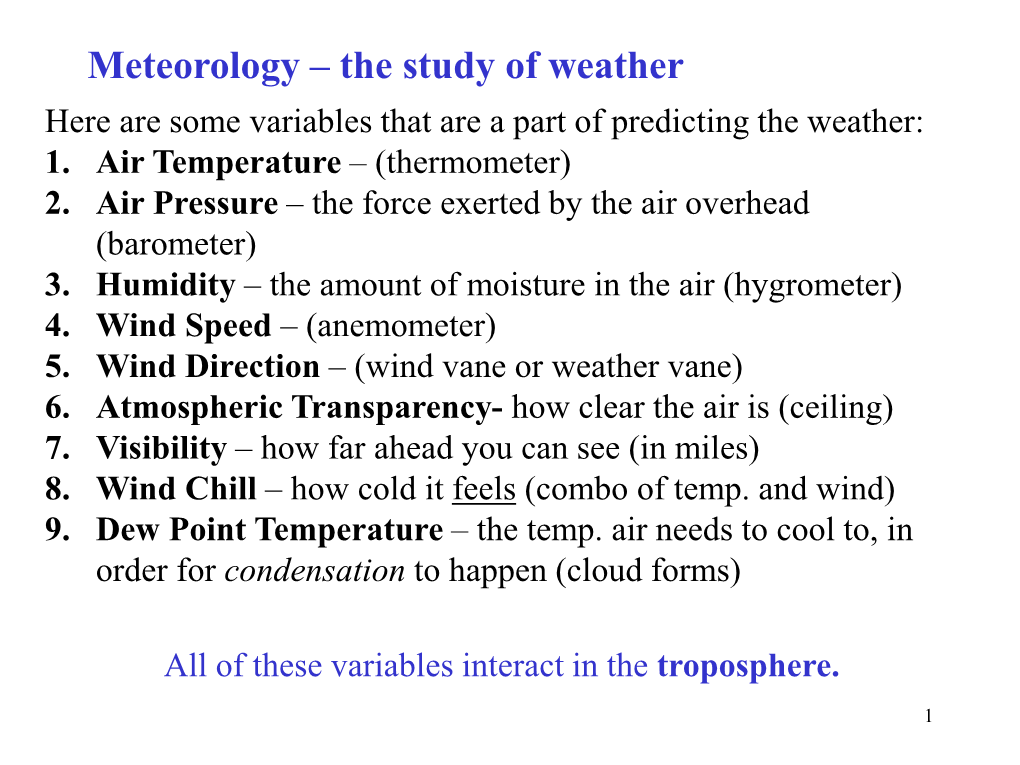
Meteorology – the Study of Weather Here Are Some Variables That Are a Part of Predicting the Weather: 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Climate Data Sources in Connecticut Patricia A
University of Connecticut OpenCommons@UConn College of Agriculture, Health and Natural Storrs Agricultural Experiment Station Resources 1-1982 Climate Data Sources in Connecticut Patricia A. Palley University of Connecticut - Storrs David R. Miller University of Connecticut - Storrs Follow this and additional works at: https://opencommons.uconn.edu/saes Part of the Climate Commons, Environmental Monitoring Commons, and the Meteorology Commons Recommended Citation Palley, Patricia A. and Miller, David R., "Climate Data Sources in Connecticut" (1982). Storrs Agricultural Experiment Station. 80. https://opencommons.uconn.edu/saes/80 Storrs Agricultural Experiment Station Bulletin 461 Climate Data Sources in Connecticut By Patricia A Palley, Assistant State Climatologist and David R. Miller, Associate Professor of Natural Resources JAN 1982 STORRS AGRICULTURAL EXPERI MENT STATION COLLEGE OF AGRICULTURE AND NATURAL RESOURCES THE UNIVERSITY OF CONNECTICUT, STORRS. CT 06268 TABLE OF CONTENTS Int roduction . 1 Types of Weather Stations 2 Parameters Measur ed 3 Summary of Climate Observations in Connecticu t 5 How to Use the Maps and Site Reports • • • • • 7 Table I Record Lengths, by parameter, of all weather stat ions i n Conn., state summary 8 Table II Record Lengths , by parame ter, of all weather stations in Conn . , by county . 9 Table III Record Lengths, by paramet er, of Nationa l Wea ther Service operat ed and coope rative stations in Conn., by county . • . 10 Table IV Re cord Lengths , by par ameter , of pr ivate data collect ors i n Conn ., by county . • . 11 Figure I Distribution of stations t hat measure rainfall . 12 Figur e II Distribution of stations t hat meas ure s nowf all . -

Handbook for the Meteorological Observation
Handbook for the Meteorological Observation Koninklijk Nederlands Meteorologisch Instituut KNMI September 2000 Contents Chapter 1. Measuring stations – General 1 Introduction 2 Variables 3 Type of observing station 4 Conditions relating to the layout of the measurement site of a weather station 5 Spatial distribution of the measuring stations and the representativeness of the observations 6 Procedures relating to the inspection, maintenance and management of a weather station 6.1 Inspection 6.2 Technical maintenance 6.3 Supervision 1. MEASURING STATIONS - GENERAL 1.1 Introduction The mission statement of the KNMI1 (from their brochure “KNMI, more than just weather” of August 1999) reads: “The KNMI is an agency with approximately five hundred employees that is part of the Ministry of Transport, Public Works and Water Management. From its position as the national knowledge centre for weather, climate and seismology, the institute is targeted entirely at fulfilling public tasks: weather forecasts and warnings monitoring the climate acquisition and supply of meteorological data and infrastructure model development aviation meteorology scientific research public information services” The tasks mentioned above are split across a number of sectors within the KNMI. One of the sectors is WM (Waarnemingen en Modellen = Observations and Models). This particular sector’s mission has been formulated as follows: “The Observations and Models sector (WM) is responsible for making the basic meteorological data available and for provision of climatological information to both internal and external users. The basic meteorological data, both current and historical, contains: - observations made by measurement, visual observation, using remote sensing or acquired from external sources - output from atmospheric and oceanographic models, acquired by processing the sector’s own models of acquired from institutes abroad. -

Lesson 6 Weather: Collecting Data GRADE 3-5 BACKGROUND Meteorologists Collect Weather Data Daily to Make Forecasts
Lesson 6 Weather: Collecting Data GRADE 3-5 BACKGROUND Meteorologists collect weather data daily to make forecasts. With the aid of high altitude weather balloons, weather equipment and gauges, satellites, and computers, accurate daily forecasts can be made. Collecting weather data in just one location and making a forecast requires a great deal of skill. Since air travels from one location to another, it is helpful to know what the approaching weather will be. In this investigation, the students will collect data for two weeks. At this time they will start seeing patterns in each of the areas. They can predict what the weather will be like the next day and for the next few days. They will also write if their predictions were correct from the previous day. Collecting the data for this lesson can be done instead of collecting the data separately in lesson 1-4. BASIC LESSON Objective(s) Students will be able to… Collect data for two weeks and use the information to detect patterns and predict weather around their location. State Science Content Standard(s) Standard 4. Students through the inquiry process, demonstrate knowledge of the composition, structures, processes and interactions of Earth's systems and other objects in space. A. 4.4 Observe and describe the water cycle and the local weather and demonstrate how weather conditions are measured. A. Record temperature B. Display data on a graph C. Interpret trends and patterns of data D. Identify and explain the use of a barometer, weather vane, and anemometer E. Collect, record and chart data from each weather instrument F. -

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Rigid Wing Sailboats: a State of the Art Survey Manuel F
Ocean Engineering 187 (2019) 106150 Contents lists available at ScienceDirect Ocean Engineering journal homepage: www.elsevier.com/locate/oceaneng Review Rigid wing sailboats: A state of the art survey Manuel F. Silva a,b,<, Anna Friebe c, Benedita Malheiro a,b, Pedro Guedes a, Paulo Ferreira a, Matias Waller c a Rua Dr. António Bernardino de Almeida, 431, 4249-015 Porto, Portugal b INESC TEC, Campus da Faculdade de Engenharia da Universidade do Porto, Rua Dr. Roberto Frias, 4200-465 Porto, Portugal c Åland University of Applied Sciences, Neptunigatan 17, AX-22111 Mariehamn, Åland, Finland ARTICLEINFO ABSTRACT Keywords: The design, development and deployment of autonomous sustainable ocean platforms for exploration and Autonomous sailboat monitoring can provide researchers and decision makers with valuable data, trends and insights into the Wingsail largest ecosystem on Earth. Although these outcomes can be used to prevent, identify and minimise problems, Robotics as well as to drive multiple market sectors, the design and development of such platforms remains an open challenge. In particular, energy efficiency, control and robustness are major concerns with implications for autonomy and sustainability. Rigid wingsails allow autonomous boats to navigate with increased autonomy due to lower power consumption and increased robustness as a result of mechanically simpler control compared to traditional sails. These platforms are currently the subject of deep interest, but several important research problems remain open. In order to foster dissemination and identify future trends, this paper presents a survey of the latest developments in the field of rigid wing sailboats, describing the main academic and commercial solutions both in terms of hardware and software. -

Reducing the Risk Runway Excursions
MAIN MENU Report . Reducing the Risk of Runway Excursions: Report of the Runway Safety Initiative . Appendixes Reducing the Risk of RUNWAY EXCURSIONS REPORT OF THE RUNWAY SAFETY INITIATIVE This information is not intended to supersede operators’ or manufacturers’ policies, practices or requirements, and is not intended to supersede government regulations. Reducing the Risk of RUNWAY EXCURSIONS REPORT OF THE RUNWAY SAFETY INITIATIVE Contents 1. Introduction 4 1.1 Definitions 4 2. Background 5 3. Data 6 Reducing the Risk of 4.0 Common Risk Factors inRunway Excursion Events 9 RUNWAY EXCURSIONS 4.1 Flight Operations 9 4.1.1 Takeoff Excursion Risk Factors 9 REPORT OF THE RUNWAY SAFETY INITIATIVE 4.1.2 Landing Excursion Risk Factors 9 4.2 Air Traffic Management 9 TABLE OF CONTENTS 4.3 Airport 9 1. Introduction .......................................................................................................................................................................... 4 1.1 Definitions ................................................................................................................................................................. 4 4.4 Aircraft Manufacturers 9 2. Background .......................................................................................................................................................................... 5 4.5 Regulators 9 3. Data ................................................................................................................................................................................... -

Product Catalog About Company
PRODUCT CATALOG ABOUT COMPANY JSC RADAR MMS Joint-stock company Radar mms is one of the global leaders in the field of developing special- and civil-purpose radio- electronic systems and suites, precision instruments, and specialized software, as well as is considered as a recognizable system integrator of avionics. JSC Radar mms was awarded the Gratitude of the President of the Russian Federation on three occasions (in 2010, 2015 and 2019) “For a great contribution to the development of the radio-electronic industry, the strengthening of the country's defense capability and labor achievements”. FOCUS AREAS І Radar Systems І Aircraft-Based Systems І Robotic Systems І Internet of Things І Specialized Software І Sensors and Measurement Systems І High-Speed Vessels and Sea-Based Systems І Services and Servicing FULL-CYCLE PRODUCTION Radar mms implements a complete cycle of research and production activities: research, development, production, testing, sales, and engineering support during the operation and maintenance. The company has its own testing facilities including the simulation and testing facility, marine testing facility, automated motion simulation system and mobile experimental laboratory, ground-based test benches and hardware-in-the-loop simulation system. The test site fully meets the needs of the company in the development, testing and production of special-purpose and civilian products. PERSONNEL QUALIFICATION Currently, the company employs more than 2500 qualified specialists, including State Prize winners, Professors, Doctors and Candidates of Science, and Associate Professors. Over 500 employees were awarded orders and medals of the Russian Federation. The company operates a number of influential schools of scientists and designers in the country and abroad. -

Weather Conditions Can Be Described As; Sunny Cloudy Windy Rainy Snowy
Grade 2 Study Guide – Science – SOL 2.6 Weather -The Earth’s weather changes continuously from day to day. Vocabulary weather- How the outside air feels and looks. -Changes in the weather are characterized by daily differences in wind, temperature, and precipitation. forecast- a prediction about what kind of weather to expect - Weather affects how we dress and the activities in which we participate. For wind- moving air that can change in speed and example, farmers keep track of the weather to know when to plant and harvest direction their crops. temperature- lower means colder and higher means warmer; measured in Fahrenheit (F) or Celsius (C) Weather Conditions can be described as; precipitation- water that falls from the sky that can be liquid or solid sunny cloudy win dy rainy snowy weather instrument- a tool that is used to measure weather water cycle – the process in which water can change from a liquid to water vapor, produce condensation, and then fall down as precipitation meteorologist- A scientist who studies and predicts the weather by measuring, observing and recording weather data. Types of Precipitation rain snow sleet hail Created by Sara Gauldin Types of Weather Instruments thermometer measures the temperature of the air rain gauge measures the amount of rain or snow that falls to the ground weather or shows the direction wind vane the wind is blowing from anemometer measures the speed Key ideas: Weather data is collected and We can measure and of the wind recorded using instruments. record weather data, This information is very useful using weather for predicting weather and instruments, including a determining weather patterns. -

In-Flight Icing Encounter and Loss of Control, Simmons Airlines, D.B.A
F PB96-91040I NTSB/AAR-96/01 DCA95MA001 NATIONAL TRANSPORTATION SAFETY BOARD WASHINGTON, D.C. 20594 AIRCRAFT ACCIDENT REPORT IN-FLIGHT ICING ENCOUNTER AND LOSS OF CONTROL SIMMONS AIRLINES, d.b.a. AMERICAN EAGLE FLIGHT 4184 AVIONS de TRANSPORT REGIONAL (ATR) MODEL 72-212, N401AM ROSELAWN, INDIANA OCTOBER 31,1994 VOLUME 1: SAFETY BOARD REPORT 6486C The National Transportation Safety Board is an independent Federal agency dedicated to promoting aviation, railroad, highway, marine, pipeline, and hazardous materials safety. Established in 1967, the agency is mandated by Congress through the Independent Safety Board Act of 1974 to investigate transportation accidents, determine the probable causes of the accidents, issue safety recommendations, study transportation safety issues, and evaluate the safety effectiveness of government agencies involved in transportation. The Safety Board makes public its actions and decisions through accident reports, safety studies, special investigation reports, safety recommendations, and statistical reviews. Information about available publications may be obtained by contacting: National Transportation Safety Board Public Inquiries Section, RE-51 490 L’Enfant Plaza, S.W. Washington, D.C. 20594 (202)382-6735 (800)877-6799 Safety Board publications may be purchased, by individual copy or by subscription, from: National Technical Information Service 5285 Port Royal Road Springfield, Virginia 22161 (703)487-4600 NTSB/AAR-96/01 PB96-910401 NATIONAL TRANSPORTATION SAFETY BOARD WASHINGTON, D.C. 20594 AIRCRAFT ACCIDENT REPORT IN-FLIGHT ICING ENCOUNTER AND LOSS OF CONTROL SIMMONS AIRLINES, d.b.a. AMERICAN EAGLE FLIGHT 4184 AVIONS de TRANSPORT REGIONAL (ATR) MODEL 72-212, N401AM ROSELAWN, INDIANA OCTOBER 31, 1994 Adopted: July 9, 1996 Notation 6486C Abstract: Volume I of this report explains the crash of American Eagle flight 4184, an ATR 72 airplane during a rapid descent after an uncommanded roll excursion. -

Doc 10057 Manual on Air Traffic Safety Electronics Personnel Competency-Based Training and Assessment
Doc 10057 Manual on Air Traffic Safety Electronics Personnel Competency-based Training and Assessment First Edition, 2017 Approved by and published under the authority of the Secretary General INTERNATIONAL CIVIL AVIATION ORGANIZATION Manual on Air Traffic Safety Electronics Personnel App B-6 Competency-based Training and Assessment SUBJECT 4: METEOROLOGY TOPIC 1: METEOROLOGY SUB-TOPIC 1.1: Introduction to meteorology 1.1.1 State the relevance of meteorology in aviation. 1 Influence on the operation of aircraft, flying conditions, aerodrome conditions. 1.1.2 State the weather prediction and measurement 1 — systems available. SUB-TOPIC 1.2: Impact on aircraft and ATS operation 1.2.1 State the meteorological conditions and their 1 e.g. Atmospheric circulation, wind, visibility, impact on aircraft operations. temperature/humidity, clouds, precipitation. 1.2.2 State the meteorological conditions hazardous to 1 e.g. Turbulence, thunderstorms, icing, aircraft operations. microbursts, squall, macro bursts, wind shear, standing water on runways (aquaplaning). 1.2.3 Explain the impact of meteorological conditions 2 e.g. Effects on equipment performance (e.g. and hazards on ATS operations. temperature inversion, rain density), increased vertical and horizontal separation, low visibility procedures, anticipation of flights not adhering to tracks, diversions, missed approaches. 1.2.4 Explain the effects of weather on propagation. 2 e.g. Anaprop, rain noise, sunspots. SUB-TOPIC 1.3: Meteorological parameters and information 1.3.1 List the main meteorological parameters. 1 Wind, visibility, temperature, pressure, humidity. 1.3.2 List the most common weather messages and 1 e.g. ICAO Annex 3. broadcasts used in aviation. Meteorology messages: TAF, METAR, SNOWTAM Broadcasts: ATIS/flight meteorology broadcast (VOLMET). -

Predicting the Weather by Sharon Fabian
Predicting the Weather By Sharon Fabian 1 If the groundhog sees his shadow there will be six more weeks of winter. Watching the groundhog is one way of predicting the weather, but it's not the most scientific way. To really forecast the weather, you will need to look at things like wind speed, wind direction, temperature, air pressure, precipitation, and humidity. 2 There are different ways to measure each of these things, and weather forecasters use all of the data to help predict the weather. 3 Temperature 4 The temperature outdoors is measured with the same type of instrument used to measure a person's temperature, a thermometer. It can be measured in either degrees Fahrenheit or degrees Celsius. 5 Wind Speed 6 Wind speed can be measured using a device called an anemometer. An anemometer is like a pinwheel turned on its side. You can make a simple one using straws and small paper cups. Arrange the straws to form an X. The middle of the X can be attached to the eraser end of a pencil with a pin; this is the axis that they will spin around. Attach a cup to the end of each straw. The cups must all face in the same direction so that the wind will spin the anemometer around the axis. You can measure how many times it revolves per minute to find the wind speed. 7 Wind speed can also be estimated by observing nature. In 1805, Sir Francis Beaufort created a scale to measure wind speed. His original scale was used to observe waves and sailing ships, but other versions of the scale can be used anywhere today. -

Dealing with the Weather Background
DEALING WITH THE WEATHER BACKGROUND DEALING WITH BACKGROUND THE WEATHER Weather affects air activity in lots of different ways. / Activity link Changes in atmospheric conditions are recorded by forecasters on charts, which help tell air activity bases “Weather measurement” about upcoming weather and how it may affect flight Answers: conditions. The changes in atmospheric conditions (A) Windsock, (B) Runway lights, (C) Instrument include temperature, precipitation (rain and snow), panel, (D) Anemometers, (E) Radar, (F) Rainfall wind speed and direction, atmospheric pressure and gauges, (G) Satellites, (H) Weather maps, (I) cloud coverage. Computerised weather maps. / Activity link Commercial aircrafts tend to fly above the weather “Weather across the UK” systems where possible, however military aircraft have to fly in all weather conditions. / Activity link There are weather-related factors that pilots need to know before each flight. These include: “Do you know your cloud types” Answers: # Wind speed and direction Cirrus - white, feathery, highest clouds. # Surface conditions Cirruscumulus - small, white patches of clouds often # Rain, snow and hail arranged in rows and present in high altitudes. # Thunderstorms Altocumulus - Altocumulus clouds are the most # Predicted changes in the weather throughout common clouds in the middle atmosphere. They their flight path look like the wool of sheep. Stratus - these hang low in the sky as a flat, There are several aids and weather measurement featureless, uniform layer of grayish cloud. They instruments that pilots use to fly safely in different look a bit like fog. weather conditions. Cumulus - these are puffy clouds that sit quite low in the sky. Stratocumulus - these are low, puffy, grayish or / Activity link whitish clouds that occur in patches with blue sky “How we measure the weather” visible in between.