Full Page Photo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

7563/11 HGN/Tt 1 DG H 2B COUNCIL of the EUROPEAN
COUNCIL OF Brussels, 10 March 2011 THE EUROPEAN UNION 7563/11 COPEN 44 EJN 20 EUROJUST 29 NOTE from: Bulgarian Permanent Representation to Delegations Subject: Council Framework Decision 2006/783/JHA of 6 October 2006 on the application of the principle of mutual recognition to confiscation orders – the Republic of Bulgaria Delegations will find enclosed the notification made by Bulgaria in relation to the abovementioned framework decision. _______________ 7563/11 HGN/tt 1 DG H 2B EN DECLARATIONS AND NOTIFICATIONS BY THE REPUBLIC OF BULGARIA IN ACCORDANCE WITH ARTICLE 3, ARTICLE 7(5) AND ARTICLE 19 OF COUNCIL FRAMEWORK DECISION 2006/783/JHA OF 6 OCTOBER 2006 ON THE APPLICATION OF THE PRINCIPLE OF MUTUAL RECOGNITION TO CONFISCATION ORDERS The Republic of Bulgaria hereby notifies the General Secretariat of the Council of the following declarations and notifications pursuant to Council Framework Decision 2006/783/JHA of 6 October 2006 on the application of the principle of mutual recognition to confiscation orders and the Law on the recognition, execution and transmission of decisions on confiscation or seizure and decisions on the enforcement of financial penalties adopted by the National Assembly of the Republic of Bulgaria on 11 February 2010 (published in the State Journal of the Republic of Bulgaria No 15 of 23 February 2010), which transposes the abovementioned Framework Decision into the law of the Republic of Bulgaria. 1) Notification pursuant to Article 3 of Framework Decision 2006/783/JHA (determination of the competent authorities): (а) When the Republic of Bulgaria is the executing State: The competent authorities with regard to the recognition of confiscation or seizure orders shall be the provincial courts and Sofia City Court. -

Annex REPORT for 2019 UNDER the “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY of the REPUBLIC of BULGAR
Annex REPORT FOR 2019 UNDER THE “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY OF THE REPUBLIC OF BULGARIA 2012 - 2020 Operational objective: A national monitoring progress report has been prepared for implementation of Measure 1.1.2. “Performing obstetric and gynaecological examinations with mobile offices in settlements with compact Roma population”. During the period 01.07—20.11.2019, a total of 2,261 prophylactic medical examinations were carried out with the four mobile gynaecological offices to uninsured persons of Roma origin and to persons with difficult access to medical facilities, as 951 women were diagnosed with diseases. The implementation of the activity for each Regional Health Inspectorate is in accordance with an order of the Minister of Health to carry out not less than 500 examinations with each mobile gynaecological office. Financial resources of BGN 12,500 were allocated for each mobile unit, totalling BGN 50,000 for the four units. During the reporting period, the mobile gynecological offices were divided into four areas: Varna (the city of Varna, the village of Kamenar, the town of Ignatievo, the village of Staro Oryahovo, the village of Sindel, the village of Dubravino, the town of Provadia, the town of Devnya, the town of Suvorovo, the village of Chernevo, the town of Valchi Dol); Silistra (Tutrakan Municipality– the town of Tutrakan, the village of Tsar Samuel, the village of Nova Cherna, the village of Staro Selo, the village of Belitsa, the village of Preslavtsi, the village of Tarnovtsi, -

Sofia Model”: Creation out of Chaos
The “Sofia Model”: Creation out of chaos Pathways to creative and knowledge-based regions ISBN 978-90-75246-62-9 Printed in the Netherlands by Xerox Service Center, Amsterdam Edition: 2007 Cartography lay-out and cover: Puikang Chan, AMIDSt, University of Amsterdam All publications in this series are published on the ACRE-website http://www2.fmg.uva.nl/acre and most are available on paper at: Dr. Olga Gritsai, ACRE project manager University of Amsterdam Amsterdam institute for Metropolitan and International Development Studies (AMIDSt) Department of Geography, Planning and International Development Studies Nieuwe Prinsengracht 130 NL-1018 VZ Amsterdam The Netherlands Tel. +31 20 525 4044 +31 23 528 2955 Fax +31 20 525 4051 E-mail: [email protected] Copyright © Amsterdam institute for Metropolitan and International Development Studies (AMIDSt), University of Amsterdam 2007. All rights reserved. No part of this publication can be reproduced in any form, by print or photo print, microfilm or any other means, without written permission from the publisher. The “Sofia Model”: Creation out of chaos Pathways to creative and knowledge-based regions ACRE report 2.10 Evgenii Dainov Ivan Nachev Maria Pancheva Vasil Garnizov Accommodating Creative Knowledge – Competitiveness of European Metropolitan Regions within the Enlarged Union Amsterdam 2007 AMIDSt, University of Amsterdam ACRE ACRE is the acronym for the international research project Accommodating Creative Knowledge – Competitiveness of European Metropolitan Regions within the enlarged Union. The project is funded under the priority 7 ‘Citizens and Governance in a knowledge-based society within the Sixth Framework Programme of the EU (contract no. 028270). Coordination: Prof. -

Global and Domestic Bulgarian Production of Cocoa and Chocolate Articles for the Period 2013-2016
Trakia Journal of Sciences, Vol. 15, Suppl. 1, pp 10-17, 2017 Copyright © 2017 Trakia University Available online at: http://www.uni-sz.bg ISSN 1313-7069 (print) ISSN 1313-3551 (online) doi:10.15547/tjs.2017.s.01.003 GLOBAL AND DOMESTIC BULGARIAN PRODUCTION OF COCOA AND CHOCOLATE ARTICLES FOR THE PERIOD 2013-2016 G. Slavova Department of Economics of Agriculture, University of Economics-Varna, Bulgaria ABSTRACT Chocolate is one of the most delicious and popular desserts on earth, but for many people around the world, it is also a serious industry. It is made from cocoa beans that grow on cocoa trees. Historians believe that chocolate consumption dates back before Columbus discovered North America, mainly in Central American societies, where it has been used for more than five millennia. Archaeological excavations prove that in Costa Rica, the Mayans drank cocoa about 400 B.C. Today, however, chocolate production and consumption is a complex commercial task that covers manufacturers and trade networks around the world. The number of cocoa miners in the world is over 14 million people. Almost 35% of the cocoa beans originate in Côte d'Ivoire. This article aims to analyze the main problems and difficulties in the world's cocoa-producing countries as well as to compare the producers, importers and exporters of cocoa and chocolate products in the European Union, including Bulgaria, by focusing on the change in production and consumption over the last years to highlight main trends and dependencies. Key words: chocolate, cocoa, analyze, cocoa-producing, import, export Chocolate is one of the most delicious and this production of cocoa grains originates from most popular dessert foods in the world, but Ivory Coast. -

Driving Restrictions, Goods Transport, 2019 Bulgaria Vehicles
Driving restrictions, goods transport, 2019 Bulgaria Vehicles concerned all goods vehicles over 15t Prohibition permanent till the end of the maintenance works Road concerned road I-1 between km 276+162 and km 282+485 Vehicles concerned all goods vehicles of over 12t total laden weight Prohibition permanent till the end of the maintenance works Road concerned road I-3 between km 193+345 (intersection with the road to Pravetz junction) and km 204+200 (intersection with I-1) Vehicles concerned all goods vehicles of over 15t total laden weight Prohibition permanent Road concerned road I-5, section Tchernootchene – Kardjali The vehicles concerned should use the following routes: · direction Haskovo – Kardjali: road I-5 – road III-505 – Manastir – road III- 507 – Voyvodino – Most – Tchiflik – Kardjali · direction Assenovgrad – Kardjali: road II-58 – road I-5, direction Haskovo – road III-505 – Manastir – road III-507 – Voyvodino – Most – Tchiflik – Kardjali · direction Mineralni Bani – Kardjali: road III-506 – road III-806 – road I-5 – road III-505 – Manastir – road III-507 – Voyvodino – Most – Tchiflik – Kardjali Vehicles concerned all goods motor vehicles of over 12t total laden weight (N3) and the towing of trailers and semi trailers with MPW over 10t (O4) Prohibition Permanent Road concerned I–5 between km 155+250 and km 184 +000 (Shipka Pass) The vehicles concerned should use the route Radnevo – road II-57 – Pet mogili – Novoselez – road II-55 – Mlekarevo – Radevo – Nova Zagora –– road I-6 – Gurkovo – Prohod na Republikata – Veliko Tarnovo. Vehicles concerned all goods vehicles of over 3.5t total laden weight Prohibition permanent till the end of the maintenance works Road concerned road I-5, section overbridge Kirkovo – Makasa Vehicles concerned all vehicles Prohibition permanent Road concerned I-7, section Varbishki Pass The vehicles concerned should use the Kotlenski Pass (I-4 road), the Rishki Pass (II-73 road) or the Prohod na Republikata Pass (road II-55). -

Youth Exchange Project
ESEI ORGANIZATION _______________________ YOUTH EXCHANGE PROJECT Project # 2020-1-BG01-KA105-078612 “Sense of the nature as a Sense of initiative - ECO entrepreneurship” co-funded by Erasmus+ Programme of the European Union Organized by ESEI - ETHNO, SOCIAL AND ECO INITIATIVES ORGANIZATION with the financial support of the “Erasmus +” programme. 29.08.2020 – 06.09.2020 Location: Rebrovo, Svoge, Sofia region Zip Code: 2294 Official language: English Duration: 9 days Number of participants: 35 Participating organizations: COMUNE DI TORINO (Italy); SEIKLEJATE VENNASKOND (Estonia), ASOCIATIA TINERILOR CU INITIATIVA CIVICA (Romania), EVROPSKE CENTRUM MLADEZE BRECLAV EUROPEAN YOUTH CENTRE BRECLAV Z. S.(Czech republic), AYUNTAMIENTO DE MURCIA (Spain), DOGAYA DONUS GENCLIK VE SPOR KULUBU DERNEGI (Turkey) Project 2020-1-BG01-KA105-078612 “Sense of the nature as a Sense of initiative - ECO entrepreneurship” co-funded by Erasmus+ Programme of the European Union ESEI ORGANIZATION _______________________ DEAR PARTICIPANT, We are looking forward to meeting you at Rebrovo this summer! Rebrovo is a small village in Svoge Municipality, Sofia Province, western Bulgaria. It is located into the mountains, in the Iskar defile. 30 km away from the capital city – Sofia. 1. What’s the idea? “Sense of the nature as a Sense of initiative - ECO entrepreneurship” is an Erasmus + Youth Mobility Project. The main objective of the project is " provoking the sense of initiative and entrepreneurship in young people, using innovative methods, based on environmental thinking". The exchange will bring together 35 young people from 7 different countries, who will share experience, ideas and knowledge in the wild. The specific objectives of the project are: - constucting a model for establishing, managing and developing a sustainable environmental organization - improving the skills and competences of young people in the sphere of entrepreneurship, innovativeness, critical thinking, making decisions, etc. -
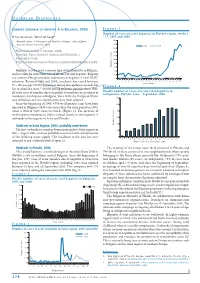
OUTBREAK DISPATCHES Hepatitis a Is the Most Common Type of Viral
O UTBREAK DISPATCHES CURRENT OUTBREAK OF HEPATITIS A IN BULGARIA, 2006 Figure 3 Number of cases of acute hepatitis in Plovdiv region, weeks 1 M Kojouharova1, Editorial team2 - 37, 2005 and 2006 1 National Center of Infectious and Parasitic Diseases, Sofi a, Bulgaria 200 2 Eurosurveillance editorial offi ce 180 2006 2005 160 Published online 5 October 2006. 140 Citation: Euro Surveill 2006;11(10):E061005.1. 120 Available from: 100 http://www.eurosurveillance.org/ew/2006/061005.asp#1 80 Number of cases 60 Hepatitis A is the most common type of viral hepatitis in Bulgaria, 40 and accounts for more than 75% of all cases of viral hepatitis. Bulgaria 20 is a country with intermediate endemicity of hepatitis A viral (HAV) 0 infection. Between 1984 and 2005, incidence has varied between 1 3 5 7 9 1113151719212325272931333537 Week number 27 – 80 cases per 100 000 population during non-epidemic periods, but Figure 4 has reached 234 cases / 100 000 during epidemic periods. Since 1983, Weekly number of cases of acute viral hepatitis in all acute cases of jaundice due to hepatitis A virus have been subject to Stolipinovo, Plovdiv, June – September 2006 mandatory notification in Bulgaria. Since 2005, the European Union 120 case definition and case classification have been adopted. Since the beginning of 2006, 4793 viral hepatitis cases have been 100 reported in Bulgaria (1498 cases more than the same period in 2005, when a total of 3295 cases occurred) (Figure 1). The increase of 80 viral hepatitis incidence in 2006 is related mainly to two hepatitis A outbreaks in the regions of Sofia and Plovdiv. -

USAID/Bulgaria List of Legacy Organizations (February 2008)
USAID/Bulgaria List of Legacy Organizations (February 2008) Governing Justly and Democratically...............................................................2 American University in Bulgaria, The (AUBG).......................................................................2 Association of Community Funds in Bulgaria (ACFB)............................................................3 Association of Danube River Municipalities (ADRM).............................................................4 Association of Rhodope Municipalities (ARM)........................................................................5 Association of South-West Municipalities................................................................................6 Broadcast Training Center Foundation (BTC) - ProMedia .....................................................7 Bulgarian Association for Alternative Dispute Resolution (BAADR)......................................8 Bulgarian Center for Not-for-Profit Law (BCNL)....................................................................9 Bulgarian Institute for Legal Reform Initiatives (BILI) .........................................................10 Center for the Study of Democracy (CSD).............................................................................11 Foundation for Local Government Reform (FLGR)...............................................................13 Legal Clinic with Angel Kunchev University, Rousse............................................................15 Legal Clinic with St. Kiril and Methodiy -

A Practical Guide for Identifying, Managing, and Monitoring of High Conservation Value Forests in Bulgaria
A practical guide for Identifying, Managing, and Monitoring of High Conservation Value Forests in Bulgaria Updated version, 2016 Prepared with the active support of ProForest on behalf of the WWF and IKEA Co-operation on Forest Projects. The updated version of the guide was prepared in the period 2014 - 2016 with the support of WWF and the working group for development for national FSC Standard for Bulgaria within a partnership of WWF and IKEA Contents Introduction of the HCVF Toolkit ................................................................................................................. 2 What are HCVs and HCV Forests? ............................................................................................................ 2 Definition of High Conservation Value Forests ......................................................................................... 2 What is the hcvf toolkit? ............................................................................................................................... 3 How was the toolkit developed? ................................................................................................................. 5 Using the toolkit ............................................................................................................................................. 6 Keys to hcvf success .................................................................................................................................... 8 HCV1. Species Diversity. .......................................................................................................................... -

Maps -- by Region Or Country -- Eastern Hemisphere -- Europe
G5702 EUROPE. REGIONS, NATURAL FEATURES, ETC. G5702 Alps see G6035+ .B3 Baltic Sea .B4 Baltic Shield .C3 Carpathian Mountains .C6 Coasts/Continental shelf .G4 Genoa, Gulf of .G7 Great Alföld .P9 Pyrenees .R5 Rhine River .S3 Scheldt River .T5 Tisza River 1971 G5722 WESTERN EUROPE. REGIONS, NATURAL G5722 FEATURES, ETC. .A7 Ardennes .A9 Autoroute E10 .F5 Flanders .G3 Gaul .M3 Meuse River 1972 G5741.S BRITISH ISLES. HISTORY G5741.S .S1 General .S2 To 1066 .S3 Medieval period, 1066-1485 .S33 Norman period, 1066-1154 .S35 Plantagenets, 1154-1399 .S37 15th century .S4 Modern period, 1485- .S45 16th century: Tudors, 1485-1603 .S5 17th century: Stuarts, 1603-1714 .S53 Commonwealth and protectorate, 1660-1688 .S54 18th century .S55 19th century .S6 20th century .S65 World War I .S7 World War II 1973 G5742 BRITISH ISLES. GREAT BRITAIN. REGIONS, G5742 NATURAL FEATURES, ETC. .C6 Continental shelf .I6 Irish Sea .N3 National Cycle Network 1974 G5752 ENGLAND. REGIONS, NATURAL FEATURES, ETC. G5752 .A3 Aire River .A42 Akeman Street .A43 Alde River .A7 Arun River .A75 Ashby Canal .A77 Ashdown Forest .A83 Avon, River [Gloucestershire-Avon] .A85 Avon, River [Leicestershire-Gloucestershire] .A87 Axholme, Isle of .A9 Aylesbury, Vale of .B3 Barnstaple Bay .B35 Basingstoke Canal .B36 Bassenthwaite Lake .B38 Baugh Fell .B385 Beachy Head .B386 Belvoir, Vale of .B387 Bere, Forest of .B39 Berkeley, Vale of .B4 Berkshire Downs .B42 Beult, River .B43 Bignor Hill .B44 Birmingham and Fazeley Canal .B45 Black Country .B48 Black Hill .B49 Blackdown Hills .B493 Blackmoor [Moor] .B495 Blackmoor Vale .B5 Bleaklow Hill .B54 Blenheim Park .B6 Bodmin Moor .B64 Border Forest Park .B66 Bourne Valley .B68 Bowland, Forest of .B7 Breckland .B715 Bredon Hill .B717 Brendon Hills .B72 Bridgewater Canal .B723 Bridgwater Bay .B724 Bridlington Bay .B725 Bristol Channel .B73 Broads, The .B76 Brown Clee Hill .B8 Burnham Beeches .B84 Burntwick Island .C34 Cam, River .C37 Cannock Chase .C38 Canvey Island [Island] 1975 G5752 ENGLAND. -

ANNUAL REPORT of FICE-BULGARIA for 2017 Contents
ANNUAL REPORT OF FICE-BULGARIA FOR 2017 Contents: 1.Introduction 2.Structural changes and members 3.Functioning 4.Strategical plans 5.FICE International 6.Conclusion Introduction Association for pedagogical and social assistance for children (APSAC) FICE-Bulgaria is a non-governmental umbrella organization, registered on May 1st 2001 under number 2987/2001 in Sofia Court of Justice. Member of FICE-Bulgaria are professionals working in field of child welfare. Its members include 39 juridical and physical persons – Homes for children deprived of parental care, social-pedagogical boarding schools, non-governmental organizations, educators, teachers, social workers, students. FICE-Bulgaria is a section of FICE-International (Federation Internationale des Communautes educatives) FICE-International comprises of more than 40 national sections - is a non-governmental organization maintaining consultative status with UNESCO, UNICEF, ECOSOC and the Council of Europe. FICE-Bulgaria is also an active member of National Network for Children in Bulgaria, the National Alliace for Social Responsibility, Coalition 2025 - network of NGOs which are actively involved in the process of deinstitutionalization of children deprived from parental care in Bulgaria. FICE-Bulgaria is participating in different expert councils of Child Protection Agency, Ministry of Education and Youth, Ministry of Labour and Social Policy. The mission of FICE-Bulgaria is to work actively for improvement the quality of life of the children of Bulgaria. FICE-Bulgaria works for: Advocacy -

Bulgaria 2000
TEAM FOR THE PREPARATION OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 National Coordinator Adviser, Human Development Strategy Unit Dr. Andrey Ivanov Gerardo Berthin Contributors Dr. Antony Todorov, Dr. Belin Mollov, Dr. Dotcho Mihaylov, Dr. Georgi Ganev, Dr. Julia Spiridonova, Dr. Mikaela Vazharova, Dr. Vassil Marinov and Luchesar Bogdanov Statistical Team Micho Chipev, Prof. Yordan Venedikov, Sergey Tzvetarski, Stoyan Tzvetkov and Todor Todorov STRATEGIC PARTNERSHIPS OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 National Statistical Institute National Center for Regional Development and Housing Policy National Association of Municipalities in the Republic of Bulgaria Friedrich Ebert Foundation, Sofia-Bulgaria ADVISORY COMMITTEE OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 Svetlana Alexandrova, New Bulgarian University; Friedrich Bauersachs, Institute for Market Economics; Bisserka Benisheva, Ministry of Foreign Affairs; Mark Bossani, Ethnic Initiative for Human Rights Foundation; Vincenzo Celeste, Embassy of Italy; Ginka Chavdarova, National Association of Municipalities; Vera Dakova, Ideas in Process; Romain Darbelley, Embassy of Switzer- land; Hristo Hristozov, European Law Society; Pentcho Houbtchev, Friedrich Ebert Foundation; Ginka Kapitanova, Foundation for Local Government Reform; Christos Makridis, European Union Delegation; Fernando Nogales, Embassy of Spain; Jorge Nieto, European Union Delegation; Ivanka Petkova, Institute for Economic Policy; Kaye Pyle, United States Agency for International Development;Valery