Marine Mammals Laboratory Manual
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Shark-Toothed Dolphin Squalodon Cetacea
Carnets Geol. 20 (2) E-ISSN 1634-0744 DOI 10.4267/2042/70716 The shark-toothed dolphin Squalodon (Cetacea: Odontoceti) from the remarkable Montagna della Majella marine vertebrate assemblage (Bolognano Formation, central Italy) Alberto COLLARETA 1 Andrea DI CENCIO 2 Renato RICCI 3 Giovanni BIANUCCI 4 Abstract: The extinct family Squalodontidae consists of heterodont, medium-sized odontocetes, featu- ring a long rostrum that houses large, procumbent incisors and heavily ornamented postcanine teeth carrying accessory denticles, hence their vernacular name, "shark-toothed dolphins". These longirostri- ne toothed whales are often seen as bridging the anatomical gap between archaic Oligocene odontoce- tes and their late Miocene to Holocene relatives. Possibly among the major marine predators of their ti- me, the shark-toothed dolphins are important components of several lower Miocene marine-mammal assemblages from the North Atlantic and Mediterranean/Paratethysian realms. In the present work, a partial skull of Squalodontidae is described from the strata of the Bolognano Formation cropping out in the northeastern sector of the Montagna della Majella massif (Abruzzo, central Italy), which has pre- viously yielded a rich lower Miocene marine-vertebrate assemblage, including eleven taxa of elasmo- branchs as well as subordinate teleosts and very fragmentary remains of marine reptiles and mam- mals. The specimen consists of the anterodorsal portion of a rostrum, preserving parts of both pre- maxillae and left maxilla, and the anteriormost seven upper left teeth. This partial skull is here identi- fied as belonging to the genus Squalodon, whose presence in the Montagna della Majella vertebrate assemblage had already been tentatively proposed on the basis of two fragmentary teeth. -

Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor
Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor: Rebecca Bailey 1 Chapter 1 The Human Body: An Orientation • Terms - Anatomy: the study of body structure and relationships among structures - Physiology: the study of body function • Levels of Organization - Chemical level 1. atoms and molecules - Cells 1. the basic unit of all living things - Tissues 1. cells join together to perform a particular function - Organs 1. tissues join together to perform a particular function - Organ system 1. organs join together to perform a particular function - Organismal 1. the whole body • Organ Systems • Anatomical Position • Regional Names - Axial region 1. head 2. neck 3. trunk a. thorax b. abdomen c. pelvis d. perineum - Appendicular region 1. limbs • Directional Terms - Superior (above) vs. Inferior (below) - Anterior (toward the front) vs. Posterior (toward the back)(Dorsal vs. Ventral) - Medial (toward the midline) vs. Lateral (away from the midline) - Intermediate (between a more medial and a more lateral structure) - Proximal (closer to the point of origin) vs. Distal (farther from the point of origin) - Superficial (toward the surface) vs. Deep (away from the surface) • Planes and Sections divide the body or organ - Frontal or coronal 1. divides into anterior/posterior 2 - Sagittal 1. divides into right and left halves 2. includes midsagittal and parasagittal - Transverse or cross-sectional 1. divides into superior/inferior • Body Cavities - Dorsal 1. cranial cavity 2. vertebral cavity - Ventral 1. lined with serous membrane 2. viscera (organs) covered by serous membrane 3. thoracic cavity a. two pleural cavities contain the lungs b. pericardial cavity contains heart c. the cavities are defined by serous membrane d. -

Module 2 : Anatomy – the Skeleton
Module 2 : Anatomy – The Skeleton In this module you will learn: The functions of the skeletal system The types of bones in the human body The effects of exercise on your bones What happens to the bones as we get older When studying to become a fitness instructor or personal trainer, you will learn all about the anatomy of the human body. Studying the skeleton is one of the foundations of your trade, you will need to know how the body is structured, the names of each bone, types of bones, importance of bone and joint health, detail of the spine and different terms of movement. Without stating the obvious, each of your clients has their own skeleton and you must be fully aware of how this works. This is for many reasons; you are a teacher and must be fully aware of how to prevent injuries, avoid unnecessary stress on the bones and, if qualified, help the client prevent or heal bone and joint related conditions or medical problems. 2.1 Understanding the Skeletal System The skeleton is comprised of 206 different bones that provide 5 main functions: Support mechanism for muscle and tissue Protection for organs Movement with bones, muscles, and joints Storing minerals and blood cells Growth Skeletal System 2.2 Bones are Formed by Ossification Some bones (such as the flat bones of your skull) in the body are formed in a similar stage to connective tissue. The process is known as direct or intramembranous ossification. Other bones are made up of cartilaginous matter, this is developed from future bone in the embryo which then dissolves and is replaced with other bone cells. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Is the Skeleton Male Or Female? the Pelvis Tells the Story
Activity: Is the Skeleton Male or Female? The pelvis tells the story. Distinct features adapted for childbearing distinguish adult females from males. Other bones and the skull also have features that can indicate sex, though less reliably. In young children, these sex-related features are less obvious and more difficult to interpret. Subtle sex differences are detectable in younger skeletons, but they become more defined following puberty and sexual maturation. What are the differences? Compare the two illustrations below in Figure 1. Female Pelvic Bones Male Pelvic Bones Broader sciatic notch Narrower sciatic notch Raised auricular surface Flat auricular surface Figure 1. Female and male pelvic bones. (Source: Smithsonian Institution, illustrated by Diana Marques) Figure 2. Pelvic bone of the skeleton in the cellar. (Source: Smithsonian Institution) Skull (Cranium and Mandible) Male Skulls Generally larger than female Larger projections behind the Larger brow ridges, with sloping, ears (mastoid processes) less rounded forehead Square chin with a more vertical Greater definition of muscle (acute) angle of the jaw attachment areas on the back of the head Figure 3. Male skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) Female Skulls Smoother bone surfaces where Smaller projections behind the muscles attach ears (mastoid processes) Less pronounced brow ridges, Chin more pointed, with a larger, with more vertical forehead obtuse angle of the jaw Sharp upper margins of the eye orbits Figure 4. Female skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) What Do You Think? Comparing the skull from the cellar in Figure 5 (below) with the illustrated male and female skulls in Figures 3 and 4, write Male or Female to note the sex depicted by each feature. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

4. Palaeontology
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Palaeontology Klug, Christian ; Scheyer, Torsten M ; Cavin, Lionel Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-113739 Conference or Workshop Item Presentation Originally published at: Klug, Christian; Scheyer, Torsten M; Cavin, Lionel (2015). Palaeontology. In: Swiss Geoscience Meeting, Basel, 20 November 2015 - 21 November 2015. 136 4. Palaeontology Christian Klug, Torsten Scheyer, Lionel Cavin Schweizerische Paläontologische Gesellschaft, Kommission des Schweizerischen Paläontologischen Abhandlungen (KSPA) Symposium 4: Palaeontology TALKS: 4.1 Aguirre-Fernández G., Jost J.: Re-evaluation of the fossil cetaceans from Switzerland 4.2 Costeur L., Mennecart B., Schmutz S., Métais G.: Palaeomeryx (Mammalia, Artiodactyla) and the giraffes, data from the ear region 4.3 Foth C., Hedrick B.P., Ezcurra M.D.: Ontogenetic variation and heterochronic processes in the cranial evolution of early saurischians 4.4 Frey L., Rücklin M., Kindlimann R., Klug C.: Alpha diversity and palaeoecology of a Late Devonian Fossillagerstätte from Morocco and its exceptionally preserved fish fauna 4.5 Joyce W.G., Rabi M.: A Revised Global Biogeography of Turtles 4.6 Klug C., Frey L., Rücklin M.: A Famennian Fossillagerstätte in the eastern Anti-Atlas of Morocco: its fauna and taphonomy 4.7 Leder R.M.: Morphometric analysis of teeth of fossil and recent carcharhinid selachiens -

Human Biology
KINDERGARTEN HUMAN BIOLOGY 2 WEEKS LESSON PLANS AND ACTIVITIES LIFE CYCLE OVERVIEW OF KINDERGARTEN ORGANISMS WEEK 1. PRE: Comparing large and small organisms. LAB: Classifying and investigating large organisms. POST: Comparing where large animals live. WEEK 2. PRE: Observing how and where organisms live. LAB: Comparing shells. POST: Comparing plants and animals. HUMAN BIOLOGY WEEK 3. PRE: Discovering the different parts of the body. LAB: Discovering and locating parts of the body. POST: Distinguishing the function of external body parts. WEEK 4. PRE: Discovering why bones are important. LAB: Comparing different models of skeletons. POST: Exploring how teeth grow. PLANT LIFE WEEK 5. PRE: Exploring how seeds grow. LAB: Planting seeds. POST: Exploring the uses of plants. WEEK 6. PRE: Defining the parts of a tree and flower. LAB: Comparing seeds and the plants they produce. POST: Comparing flowers, stems, and leaves. NATURAL ENVIRONMENT WEEK 7. PRE: Exploring land and marine environments. LAB: Comparing and contrasting environments. POST: Describing a favorite environment. WEEK 8: PRE: Exploring the order of a natural community. LAB: Dramatizing who eats whom. POST: Characterizing producers and consumers. Math/Science Nucleus ©1990,2000 2 LIFE CYCLE - HUMAN BIOLOGY (KA) PRE LAB Students look at pictures of the body and locate body organs. OBJECTIVES: 1. Discovering organs inside the body. 2. Labeling body parts. VOCABULARY: heart kidney lung muscle organ skeleton stomach MATERIALS: Look Inside Your Body by Gina Ingoglia (Putnam) Human Body Placemats BACKGROUND: A child's body grows every day and unless someone points out the changes, it is difficult for that child to understand what is happening. -

Proceedings of the United States National Museum
DESCRIPTION OF TWO SQUALODONTS RECENTLY DIS- COVERED IN THE CALVERT CLIFFS, ^LVRYLAND; AND NOTES ON THE SHARK-TOOTHED CETACEANS. By Remington Kellogg, Of the Bureau of Biological Survey, United States Departynent of Agriculture. INTRODUCTION. When the study of paleontology was less advanced than it is at present, any announcement of the discovery of prehistoric animals unlike the living indigenous fauna was usually received with some suspicion. In spite of this distrust, many attempts were made by the early writers to explain the presence of fossil remains of marine mammals in strata above the sea level or at some distance from the seashore. In one of these treatises ' is found what is, apparently, the first notice of the occurrence of fossil remains referable to shark- toothed cetaceans. The author of this work figures a fragment of a mandible, obtained from the tufa of Malta, which possesses three serrate double-rooted molar teeth. Squalodonts as such were first brought to the attention of ]mleon- tologists in 1840, by J. P. S. Grateloup, ' a French naturalist primarily interested in marine invertebrates. In the original description of Squalodon, Grateloup held the view that this rostral fragment from Leognan, France, belonged to some large saurian related to Iguanodon. Following the anouncement of this discovery. Von Meyer ^ called at- tention to the teeth of this fossil, and concluded that the specimen was referable to some flesh-eating cetacean. A year later, Grateloup* concurred in this opinion. One other specimen should be mentioned in this connection, but, unfortunately, its relationships are quite uncer- tain. This specimen consists of a single tooth which was thought to bear resemblance to some pinniped by Von Meyer ^ and named Pachy- odon mirahilis. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Whale Evolution: a Whale of a Tale
Creation Research Society Quarterly 2012. 49:122–134. 122 Creation Research Society Quarterly Whale Evolution: A Whale of a Tale Jerry Bergman* Abstract review of the evolution of whales from terrestrial land animals finds A that the evidence used to support the current theory is either wrong or very questionable. A focus is on the hip bone and fetal teeth evidence because they are commonly used as proof for the land mammal-to-whale evolution theory. The putative fossil evidence for whale evolution from terrestrial animals is also evaluated, concluding that the examples used are likely all extinct mammals and not transitional forms. Introduction be only about 20 feet long, but baleen A major problem has been deter- The term “whale” is a common noun and blue whales can grow up to 100 feet mining which terrestrial animal whales that can refer to all marine mammals long. Toothed whales are, on average, evolved from. Charles Darwin proposed called cetaceans (members of order smaller then baleen whales, ranging one of the first theories of whale evolu- cetacea), including dolphins and por- from 3 to 32 feet long, although most tion, suggesting they evolved from bears. poises. In this paper the term “whales” are from 10 to 30 feet long. Blue whales He wrote, “I can see no difficulty in a excludes both dolphins and porpoises. can weigh up to 150 tons. race of bears being rendered, by natural Classification of whales divides them selection, more and more aquatic in into two groups; toothed whales and their structure and habits, with larger baleen whales, the latter of which use The Origin of Whales and larger mouths, till a creature was large brush-like structures to filter food The evolution of whales is one of the produced as monstrous as a whale” from the ocean. -
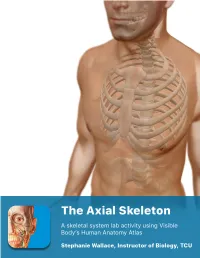
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled.