EPA Method 905.0: Radioactive Strontium in Drinking Water (PDF)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Strontium Nitrate
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Strontium nitrate ≥99 %, p.a., ACS article number: 4413 date of compilation: 2016-10-04 Version: 2.0 en Revision: 2018-07-19 Replaces version of: 2016-10-04 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Identification of the substance Strontium nitrate Article number 4413 Registration number (REACH) This information is not available. EC number 233-131-9 CAS number 10042-76-9 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: laboratory chemical laboratory and analytical use 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet e-mail (competent person) : [email protected] 1.4 Emergency telephone number Emergency information service Poison Centre Munich: +49/(0)89 19240 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 2.14 oxidising solid (Ox. Sol. 1) H271 3.3 serious eye damage/eye irritation (Eye Dam. 1) H318 2.2 Label elements Labelling according to Regulation (EC) No 1272/2008 (CLP) Signal word Danger Ireland (en) Page 1 / 15 Safety data sheet according to Regulation (EC) No. -

Improvement of High−Temperature Characteristics of the Sintered
686 EJ60ケooho勿zゑsψりノ 一Article一 Improvement of High-Temperature Characteristics of the Sintered Nickel Positive Electrode for an Alkaline Storage Battery Katsuhiko SHINYAMA,*Yoshifumi MAGARI,Atsuhiro FUNAHASHI, Toshiyuki NOHMA,and Ikuo YONEZU R&D Business Unit,Mobile Energy Company,Sanyo Electric Co.,Ltd.(7-3-21bukidai-higashimachi,Nishi-ku,Kobe City,Hyogo651-2242,Japan) Received August9,2002;Accepted May20,2003 The high-temperature characteristics of sintered nickel positive electrodes for alkaline storage batteries such as nickel・metal hydride batteries and nicke1-cadmium batteries were investigated.Generally,the discharge capacity of an alkaline storage battery charged at high temperature is smaller than that charged at room temperature due to the oxygen evolution reaction.In order to enhance high-temperature characteristics,we coated sintered n量ckel positive electrodes with yttrium hydroxide,calcium hydroxide or cobalt hydroxide.Th6high-temperature characteristics of the sintered nickel positive electrodes coated with y血ium hydroxide and calcium hydroxide by immersing them in sodium hydroxide solution after immersing in nitrate solution were greatly enhanced because of the increased oxy. gen OVerVOltage. κ¢y防鳩:Sintered Nickel Positive Electrode,High-temperature Characteristics,Alkaline Storage Battery,Oxygen Overvoltage l lntroduction In the past,the electrolyte composition3)and dissolv- Cun℃ntly,alkaline storage batteries such as nicke1- ing such elements as cobalt and calcium into nickel hy- cadmium batteries and nickel-metal hydride -

Rapid Coprecipitation Technique Using Yttrium Hydroxide for the Preconcentration and Separation of Trace Elements in Saline Water Prior to Their ICP-AES Determination
ANALYTICAL SCIENCES AUGUST 2007, VOL. 23 1021 2007 © The Japan Society for Analytical Chemistry Notes Rapid Coprecipitation Technique Using Yttrium Hydroxide for the Preconcentration and Separation of Trace Elements in Saline Water Prior to Their ICP-AES Determination Shigehiro KAGAYA,† Satoshi MIWA, Toshiyuki MIZUNO, and Koji TOHDA Graduate School of Science and Engineering for Research, University of Toyama, Gofuku 3190, Toyama 930–8555, Japan Yttrium hydroxide quantitatively coprecipitated Be(II), Ti(IV), Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Cd(II), and Pb(II) at pH 9.6 – 10.0 for seawater and pH 10.5 – 11.4 for a table-salt solution. The coprecipitated elements could be determined by inductively coupled plasma atomic emission spectrometry; yttrium was used as an internal standard element. The detection limits ranged from 0.0016 μg (Mn(II)) to 0.22 μg (Zn(II)) in 100 mL of sample solutions. The operation time required to separate 11 elements was approximately 30 min. (Received May 25, 2007; Accepted June 18, 2007; Published August 10, 2007) It is important to determine trace elements in environmental of many elements. However, to our knowledge, no application samples from the viewpoint of environmental management and/or of yttrium hydroxide to the preconcentration/separation of trace protection from hazardous infection1 as well as comprehension elements in environmental water samples has been reported. of the distribution of trace elements in the environment.2 In this work, we developed a preconcentration/separation Recently, multielement profiling analysis, in which major-to- method based on a rapid coprecipitation technique using yttrium ultratrace elements are determined and the distribution patterns hydroxide as a coprecipitant for the determination of trace of the elements are analyzed, has gathered attention to provide elements in environmental water samples, especially saline information about the elemental cycle in the environment.3,4 water. -

Evaluation of Anti-Corrosion and Anti-Galling Performance
EVALUATION OF ANTI-CORROSION AND ANTI-GALLING PERFORMANCE OF A NOVEL GREASE COMPOUND A Thesis by JOHN OLUMIDE REIS Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Hong Liang Committee Members, Chii-Der Suh Sevan Goenezan Head of Department, Andreas A. Polycarpou December 2017 Major Subject: Mechanical Engineering Copyright 2017 John Olumide Reis ABSTRACT In this research, the effectiveness of CeO2, Y2O3 and Al2O3 as anti-corrosion and anti-wear additives in commercial grease was investigated. An experimental approach was used to carry on the research. The weight loss of steel coupons protected with a layer of thin grease, with and without the anti-corrosion additives in a corrosive environment was determined. The Friction factor of the grease compound was also evaluated. The accelerated corrosion tests were performed in a salt spray chamber for an exposure time of 2 weeks (336 hours). The corrosive medium was 5 % wt. of Brine. By varying the weight compositions of the additives (1% wt. and 3% wt.), and comparing the corrosion rates with that of base grease, the effectiveness of various grease additives was evaluated. The result showed that corrosive losses of the test samples can be effectively reduced by adding relatively small amounts of CeO2, Y2O3 and Al2O3 to the base grease. Corroded surfaces were examined using the Optical microscope to clarify the corrosion mechanism. The friction test was carried out using a galling tester. The standard test procedure as specified in API RP 7A1 was followed. -

Strontium Chromate from Austria and France
Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 U.S. International Trade Commission Washington, DC 20436 U.S. International Trade Commission COMMISSIONERS David S. Johanson, Chairman Irving A. Williamson Meredith M. Broadbent Rhonda K. Schmidtlein Jason E. Kearns Catherine DeFilippo Director of Operations Staff assigned Kristina Lara, Investigator Samantha DeCarlo, Industry Analyst Tana von Kessler Economist Jennifer Brinckhaus, Accountant KaDeadra McNealy, Statistician David Goldfine, Attorney Douglas Corkran, Supervisory Investigator Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 CONTENTS Page ....................................................................................................................... 1 ........................................................................................................ 3 Introduction ................................................................................................................ I‐1 Background ................................................................................................................................ I‐1 Statutory criteria and organization of the report .................................................................... -

Strontium-90 and Strontium-89: a Review of Measurement Techniques in Environmental Media
STRONTIUM-90 AND STRONTIUM-89: A REVIEW OF MEASUREMENT TECHNIQUES IN ENVIRONMENTAL MEDIA Robert J. Budnitz Lawrence Berkeley Laboratory University of California Berkeley, CA 91*720 1. IntroiUiction 2. Sources of Enivronmental Radiostrontium 3. Measurement Considerations a. Introduction b. Counting c. •»Sr/"Sr Separation 4. Chemical Techniques a. Air b. Water c. Milk d. Other Media e. Yttrium Recovery after Ingrowth f. Interferences S- Calibration Techniques h. Quality Control 5. Summary and Conclusions 6. Acknowli'J.jnent 7. References -NOTICE- This report was prepared as an account of worK sponsored by the United States Government. Neither the United States nor the United Slates Atomic Energy Commission, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any wananty, express or implied, or asjumes any legal liabili:y or responsibility for the accuracy, com pleteness or usefulness of any Information, apparatus, J product or process disclosed, or represents that its use I would not infringe privately owned rights. Mmh li\ -1- 1. INTRODUCTION There are only two radioactive isotopes SrflO of strontium of significance in radiological measurements in the environment: strontium-89 Avg.jS energy 196-1 k«V and strontium-90. 100 Strontium-90 is the more significant from the point of view of environmental impact. 80 Energy keV 644 This is due mostly to its long half-life % Emission 100 (28.1 years). It is a pure beta emitter with 60 Typ* of only one decay mode, leading to yttrium-90 •mission by emission of a negative beta with HUBX * 40 h S46 keV. -

PUBLIC HEALTH STATEMENT Strontium CAS#: 7440-24-6
PUBLIC HEALTH STATEMENT Strontium CAS#: 7440-24-6 Division of Toxicology April 2004 This Public Health Statement is the summary External exposure to radiation may occur from chapter from the Toxicological Profile for natural or man-made sources. Naturally occurring strontium. It is one in a series of Public Health sources of radiation are cosmic radiation from space Statements about hazardous substances and their or radioactive materials in soil or building materials. health effects. A shorter version, the ToxFAQs™, is Man-made sources of radioactive materials are also available. This information is important found in consumer products, industrial equipment, because this substance may harm you. The effects atom bomb fallout, and to a smaller extent from of exposure to any hazardous substance depend on hospital waste and nuclear reactors. the dose, the duration, how you are exposed, personal traits and habits, and whether other If you are exposed to strontium, many factors chemicals are present. For more information, call determine whether you’ll be harmed. These factors the ATSDR Information Center at 1-888-422-8737. include the dose (how much), the duration (how _____________________________________ long), and how you come in contact with it. You must also consider the other chemicals you’re This public health statement tells you about exposed to and your age, sex, diet, family traits, strontium and the effects of exposure. lifestyle, and state of health. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in 1.1 WHAT IS STRONTIUM? the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for Strontium is a natural and commonly occurring long-term federal cleanup activities. -

Electrochemical Separation and Purification of Yttrium-86
Radiochim. Acta 90, 225–228 (2002) by Oldenbourg Wissenschaftsverlag, München Electrochemical separation and purification of yttrium-86 By G. Reischl1,F.Rösch2 and H.-J. Machulla1 ,∗ 1 PET-Center, Division of Radiopharmacy, University Hospital Tübingen, D-72076 Tübingen, Germany 2 Institute of Nuclear Chemistry, University of Mainz, D-55099 Mainz, Germany (Received July 5, 2001; accepted in revised form October 24, 2001) Yttrium-86 / Electrolytic purification / radioactive rare earth nuclides onto a small tungsten cath- Electrochemical separation / Y-86 labeling ode was described in 1974 [6]. Recently, the electrochemical deposition of radionuclides of superheavy elements in de- pendence on electrode material and deposition potential was Summary. For quantitative determination of in vivo dosime- investigated [7]. An electrochemical method, based on a pro- try of 90Y-labeled radiotherapeuticals by means of PET, the cedure developed at Mainz [8, 9] to isolate 90Sr from a mix- positron emitting analogue yttrium-86 was produced at a low ture of 90Yand90Sr (c.a.) electrochemically, appeared very 86 86 energy (“medical”) cyclotron via the known Sr(p, n) Y 86 86 promising for isolating and purifying Y. reaction. Using 200 mg of SrCO3 (enrichment 95.6%) and protons of 15.1 MeV energy, average yields of 86Y of 48 ± 8MBq/µA h were produced. After dissolution of 86 86 Experimental SrCO3 in 3 ml of 0.6 N HNO3, Y was deposited in a simple and highly efficient electrochemical two-step procedure onto Materials a platinum cathode at 450 mA (= 20 mA/cm2). The isotope was finally removed from the electrode by 100–300 µlof Strontium-86 was supplied by Chemotrade, Germany, with . -
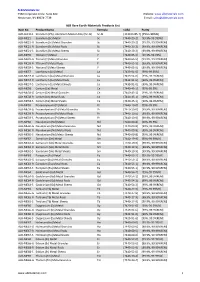
ALB Materials Inc Product List.Xlsx
ALB Materials Inc 2360 Corporate Circle. Suite 400 Website: www.albmaterials.com Henderson, NV 89074-7739 E-mail: [email protected] ALB Rare Earth Materials Products List Item No. Product Name Formula CAS# Purity ALB-AL2113 Scandium (2%)- Aluminum Master Alloy (Sc-Al) Sc-Al [113413-85-7] [2%Sc+98%Al] ALB-ME21 Scandium (Sc) Metal Sc [7440-20-2] [99.9%-99.999%] ALB-ME21-G Scandium (Sc) Metal Granules Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-R Scandium (Sc) Metal Rods Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-S Scandium (Sc) Metal Sheets Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME39 Yttrium (Y) Metal Y [7440-65-5] [99.9%-99.99%] ALB-ME39-G Yttrium (Y) Metal Granules Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-R Yttrium (Y) Metal Rods Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-S Yttrium (Y) Metal Sheets Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME57 Lanthanum (La) Metal La [7439-91-0] [99%-99.95%] ALB-ME57-G Lanthanum (La) Metal Granules La [7439-91-0] [99%, 99.9%REM] ALB-ME57-R Lanthanum (La) Metal Rods La [7439-91-0] [99%, 99.9%REM] ALB-ME57-S Lanthanum (La) Metal Sheets La [7439-91-0] [99%, 99.9%REM] ALB-ME58 Cerium (Ce) Metal Ce [7440-45-1] [99%-99.9%] ALB-ME58-G Cerium (Ce) Metal Granules Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-R Cerium (Ce) Metal Rods Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-S Cerium (Ce) Metal Sheets Ce [7440-45-1] [99%, 99.9%REM] ALB-ME59 Praseodymium (Pr) Metal Pr [7440-10-0] [99%-99.9%] ALB-ME59-G Praseodymium (Pr) Metal Granules Pr [7440-10-0] [99.9%, 99.99%REM] ALB-ME59-R Praseodymium (Pr) Metal Rods Pr [7440-10-0] -

Strontium Carbonate Solubility Data in Aqueous Mixtures of Monoethyleneglycol Under a Carbon Dioxide Atmosphere
Brazilian Journal ISSN 0104-6632 of Chemical Printed in Brazil www.scielo.br/bjce Engineering Vol. 35, No. 02, pp. 395 - 402, April - June, 2018 dx.doi.org/10.1590/0104-6632.20180352s20160355 STRONTIUM CARBONATE SOLUBILITY DATA IN AQUEOUS MIXTURES OF MONOETHYLENEGLYCOL UNDER A CARBON DIOXIDE ATMOSPHERE Deborah C. de Andrade1,*, Naíra S. de A. Maniçoba1, Rony O. Sant'ana1, Fedra A.V. Ferreira1, Camila S. Figueiredo2, Jailton F. Nascimento2, Leonardo S. Pereira2 and Osvaldo Chiavone-Filho1 1UFRN. Campus Universitário. Departamento de Engenharia Química. Lagoa Nova. Natal – RN – Brazil, ZIP 59066-800. 2PETROBRAS/CENPES/PDEP/TPP. Av. Horácio Macedo, 950 – Cidade Universitária - Ilha do Fundão. Rio de Janeiro – RJ – Brazil, ZIP 219141-915. (Submitted: June 1, 2016; Revised: March 20, 2017; Accepted: April 5, 2017) Abstract - In order to inhibit natural gas hydrate formation, monoethyleneglycol (MEG) is usually injected into producing well heads. The MEG regeneration process is continuously performed at the platform. Scaling problems usually occur due to the presence of chlorides and carbonates. This work presents salt solubility data for the aqueous system with strontium carbonate, MEG and carbon dioxide. A specific analytical method was developed. Thus, experimental data for strontium carbonate (SrCO3) solubility at various carbon dioxide pressures are reported. Solubilities of SrCO3 in water were measured from 760 to 1610 mmHg at 278.15. 288.15. 298.15 and 323.15 K. For the mixed solvent (ms) conditions, solubilities were measured at 298.15 K and four isobars, i.e., 760, 1210, 1410 and 1520 mmHg. Experimental data were correlated and demonstrated to be accurate for thermodynamic modeling and process simulation. -

Method for Producing Strontium Nitrate
Patentamt JEuropâischesEuropean Patent Office (H) Publication number: O 025 237 Office européen des brevets Bl (12 EUROPEAN PATENT SPECIFICATION (45) Dateof publication of patent spécification: 16.02.83 @ Int. Cl.3: C 01 F 11/38 @ Application number: 80200738.5 (22) Date offiling: 04.08.80 (54) Method for producing strontium nitrate. (30) Priority: 13.08.79 US 66117 (73) Proprietor: FMC Corporation 2000 Market Street (43) Date of publication of application : Philadelphia Pennsylvanie 19103 (US) 18.03.81 Bulletin 81/11 (72) Inventor: Sansone, Michael John (45) Publication of the grantof the patent: 17 James Street 16.02.83 Bulletin 83/7 Shoreham New York 1 1 786 (US) Inventor: Manganaro, James Lawrence @ Designated Contracting States: 3 Buxton Drive BE DE FR GB IT NL East Windsor New Jersey 08520 (US) (56) Références cited: (74) Représentative: Plucker, Guy et al, US-A-3010788 OFFICE KIRKPATRICK 4 Square de Meeûs CHEMICAL & METALLURGICAL ENGINEERING B-1 040 Bruxelles (BE) January 1946 "Strontium Chemicals" pages 152 to 155. Chemical Abstracts vol. 85, November 1976 Columbus, Ohio, USA. D.L. STEIN "Extraction of strontium values from celestite concentrate at the Kaiser plant in Nova Scotia" page 214, column 2, abstract no. 146349x. GMELINS HANDBUCH DER ANORGANISCHEN CHEMIE, 8th édition 1960, VERLAG CHEMIE. Weinheim supplementary volume "Strontium" pages 174, 184 to 185. Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. -

Zirconia-Ceria-Yttria-Based Mixed Oxide and Process for Producing The
(19) TZZ_ _Z_T (11) EP 1 921 044 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C01G 25/00 (2006.01) B01J 23/10 (2006.01) 28.02.2018 Bulletin 2018/09 B01D 53/94 (2006.01) B01J 21/06 (2006.01) B01J 35/10 (2006.01) (21) Application number: 07118246.3 (22) Date of filing: 10.10.2007 (54) Zirconia-ceria-yttria-based mixed oxide and process for producing the same Auf Zirkonium-Cerium-Yttrium basierendes Mischoxid und Herstellungsverfahren dafür Oxyde mixte à base de zirconium-cérium-yttrium et son procédé de fabrication (84) Designated Contracting States: • Kodama, Hiroshi DE FR GB Osaka 5590025 (JP) (30) Priority: 12.10.2006 JP 2006305934 (74) Representative: Manley, Nicholas Michael 08.08.2007 JP 2007206394 WP Thompson 8th Floor (43) Date of publication of application: 1 Mann Island 14.05.2008 Bulletin 2008/20 Liverpool L3 1BP (GB) (73) Proprietor: Daiichi Kigenso Kagaku Kogyo Co., (56) References cited: Ltd. EP-A- 1 006 081 EP-A- 1 035 074 Osaka-shi, EP-A- 1 894 620 WO-A-2007/093593 Osaka 559-0025 (JP) JP-A- 2003 277 059 US-B1- 6 387 338 (72) Inventors: • Okamoto, Hiroshi Osaka 5590025 (JP) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid.