A Three-Dimensional Thalamocortical Dataset for Characterizing Brain Heterogeneity: X-Ray Microct Images (Tif)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

Conditional Ablation of Brain-Derived Neurotrophic Factor-Trkb Signaling Impairs Striatal Neuron Development
Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development Yun Lia,1, Daishi Yuia, Bryan W. Luikarta,2, Renée M. McKaya, Yanjiao Lia, John L. Rubensteinb, and Luis F. Paradaa,3 aDepartment of Developmental Biology and Kent Waldrep Center for Basic Research on Nerve Growth and Regeneration, University of Texas Southwestern Medical Center, Dallas, TX 75390; and bNina Ireland Laboratory of Developmental Neurobiology, Department of Psychiatry, University of California, San Francisco, CA 94143 Contributed by Luis F. Parada, August 2, 2012 (sent for review June 21, 2012) Neurotrophic factors, such as brain-derived neurotrophic factor cascades pertaining to development, maturation, and function of (BDNF), are associated with the physiology of the striatum and the striatum remains to be delineated. the loss of its normal functioning under pathological conditions. In this study, we conditionally ablated BDNF or its receptor The role of BDNF and its downstream signaling in regulating the TrkB in corticostriatal and nigrostriatal neuronal circuits. We development of the striatum has not been fully investigated, found that Bdnf deletion in both cortex and substantia nigra led to Bdnf complete depletion of BDNF protein in the striatum. Mutant mice however. Here we report that ablation of in both the cortex displayed dramatic developmental abnormalities and neurological and substantia nigra depletes BDNF in the striatum, and leads to impairments. Furthermore, specific deletion of TrkB from striatal fi impaired striatal development, severe motor de cits, and postnatal neurons was sufficient to produce this wide range of developmental lethality. Furthermore, striatal-specific ablation of TrkB, the gene deficits. Thus, our results demonstrate that BDNF and TrkB play encoding the high-affinity receptor for BDNF, is sufficient to elicit critical paracrine and cell-autonomous roles, respectively, in the an array of striatal developmental abnormalities, including de- development and maintenance of striatal neurons. -

Injection of a Dopamine Type 2 Receptor Antagonist Into the Dorsal Striatum Disrupts Choices Driven by Previous Outcomes, but Not Perceptual Inference
6298 • The Journal of Neuroscience, April 22, 2015 • 35(16):6298–6306 Behavioral/Cognitive Injection of a Dopamine Type 2 Receptor Antagonist into the Dorsal Striatum Disrupts Choices Driven by Previous Outcomes, But Not Perceptual Inference X Eunjeong Lee, Moonsang Seo, Olga Dal Monte, and Bruno B. Averbeck Laboratory of Neuropsychology, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland 20892-4415 Decisions are often driven by a combination of immediate perception and previous experience. In this study, we investigated how these two sources of information are integrated and the neural systems that mediate this process. Specifically, we injected a dopamine type 1 antagonist (D1A; SCH23390) or a dopamine type 2 antagonist (D2A; eticlopride) into the dorsal striatum while macaques performed a task in which their choices were driven by perceptual inference and/or reinforcement of past choices. We found that the D2A affected choices based on previous outcomes. However, there were no effects of the D2A on choices driven by perceptual inference. We found that the D1A did not affect perceptual inference or reinforcement learning. Finally, a Bayesian model applied to the results suggested that the D2Amaybeincreasingnoiseinthestriatalrepresentationofvalue,perhapsbydisruptingthestriatalpopulationthatnormallyrepresents value. Key words: action value; dorsal striatum; neuromodulation; Parkinson’s disease; reinforcement learning; sequential decision making Introduction available in the current trial to drive the choice. Reinforcement Decisions are often driven by a combination of immediate per- learning or learning from past outcomes has often been attrib- ceptual evidence and previous experience. Several groups have uted to plasticity within the striatum (Graybiel, 2008; Cockburn studied perceptual decision-making tasks, in which stochastic et al., 2014). -

Age-Related Change in 5-HT6 Receptor Availability in Healthy Male Volunteers Measured with 11C-GSK215083 PET
BRIEF COMMUNICATION Age-Related Change in 5-HT6 Receptor Availability in Healthy Male Volunteers Measured with 11C-GSK215083 PET Rajiv Radhakrishnan1, Nabeel Nabulsi2, Edward Gaiser1, Jean-Dominique Gallezot2, Shannan Henry2, Beata Planeta2, Shu-fei Lin2, Jim Ropchan2, Wendol Williams1, Evan Morris2,3, Deepak Cyril D’Souza1, Yiyun Huang2, Richard E. Carson2,3, and David Matuskey1,2 1Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut; 2Department of Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, Connecticut; and 3Department of Biomedical Engineering, Yale University, New Haven, Connecticut because it presents an attractive therapeutic target for neuropsychi- Serotonin receptor 6 (5-hydroxytrypamine-6, or 5-HT6) is a potential atric disorders. therapeutic target given its distribution in brain regions that are Functionally, 5-HT6 exhibits excitatory action, but it can also important in depression, anxiety, and cognition. This study sought colocalize with g-aminobutyric acid–ergic neurons and produce an to investigate the effects of age on 5-HT6 receptor availability using inhibition of brain activity leading to complicated and discrepant 11 C-GSK215083, a PET ligand with affinity for 5-HT6 in the striatum results (2,4). Heterogeneous effects are also seen with other neu- and 5-HT in the cortex. Methods: Twenty-eight healthy male vol- 2A rotransmitters in specific brain regions, with 5-HT antagonism unteers (age range, 23–52 y) were scanned with 11C-GSK215083 6 PET. Time–activity curves in regions of interest were fitted using a resulting in increased extracellular glutamate, dopamine, and ace- multilinear analysis method. Nondisplaceable binding potential tylcholine in the frontal cortex and hippocampus (5,6), whereas (BPND) was calculated using the cerebellum as the reference region 5-HT6 agonists have been shown to produce increased extracellu- and corrected for partial-volume effects. -

Neuromodulation Shapes Interneuron Communication in the Mouse Striatum
From DEPARTMENT OF NEUROSCIENCE Karolinska Institutet, Stockholm, Sweden NEUROMODULATION SHAPES INTERNEURON COMMUNICATION IN THE MOUSE STRIATUM Matthijs Constantijn Dorst Stockholm 2020 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by US-AB © Matthijs Constantijn Dorst, 2020 ISBN 978-91-7831-908-4 Neuromodulation shapes interneuron communication in the mouse Striatum THESIS FOR DOCTORAL DEGREE (Ph.D.) By Matthijs Constantijn Dorst Principal Supervisor: Opponent: Professor Gilad Silberberg Professor Hagai Bergman Karolinska Institutet The Hebrew University of Jerusalem Department of Neuroscience Edmond & Lily Safra Center for Brain Sciences Co-supervisor(s): Examination Board: Professor Per Uhlén Professor Per Svenningsson Karolinska Institutet Karolinska Institutet Department of Medical Biochemistry and Department of Clinical Neuroscience Biophysics Division of Neuropharmacology - movement disorders Senior lecturer Karima Chergui Karolinska Institutet Department of Physiology and Pharmacology Division of Molecular Neurophysiology Professor Klas Kullander Uppsala Universitet Department of Neuroscience Research group Formation and Function of Neuronal Circuits Included Studies The following studies are included in this thesis, and will be referenced through- out the text as such: Study 1 Garas, F.N., Shah, R.S., Kormann, E., Doig, N.M., Vinciati, F., Nakamura, K.C., Dorst, M.C., Smith, Y., Magill, P.J. and Sharott, A., 2016. Sec- retagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife, 5, p.e16088. Study 2 Lindroos, R., Dorst, M.C., Du, K., Filipović, M., Keller, D., Ketzef, M., Kozlov, A.K., Kumar, A., Lindahl, M., Nair, A.G., Pérez-Fernández, J., Grillner, S., Silberberg, G., Kotaleski, J.H., 2018. Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales—Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4. -

Motor Systems Basal Ganglia
Motor systems 409 Basal Ganglia You have just read about the different motor-related cortical areas. Premotor areas are involved in planning, while MI is involved in execution. What you don’t know is that the cortical areas involved in movement control need “help” from other brain circuits in order to smoothly orchestrate motor behaviors. One of these circuits involves a group of structures deep in the brain called the basal ganglia. While their exact motor function is still debated, the basal ganglia clearly regulate movement. Without information from the basal ganglia, the cortex is unable to properly direct motor control, and the deficits seen in Parkinson’s and Huntington’s disease and related movement disorders become apparent. Let’s start with the anatomy of the basal ganglia. The important “players” are identified in the adjacent figure. The caudate and putamen have similar functions, and we will consider them as one in this discussion. Together the caudate and putamen are called the neostriatum or simply striatum. All input to the basal ganglia circuit comes via the striatum. This input comes mainly from motor cortical areas. Notice that the caudate (L. tail) appears twice in many frontal brain sections. This is because the caudate curves around with the lateral ventricle. The head of the caudate is most anterior. It gives rise to a body whose “tail” extends with the ventricle into the temporal lobe (the “ball” at the end of the tail is the amygdala, whose limbic functions you will learn about later). Medial to the putamen is the globus pallidus (GP). -

Direct Visualization and Characterization of The
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.25.008318; this version posted March 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Direct visualization and characterization of the 2 human zona incerta and surrounding structures 3 Running Title: Jonathan C. Lau1,2,3,4,*, Yiming Xiao2,3, Roy A.M. Haast2,3, Greydon Gilmore1,4, Direct visualization Kamil Uludag7,8,9, Keith W. MacDougall1, Ravi S. Menon2,3,6, of the zona incerta 1 1,2,3,4,6,& 1,2,3,4,5,6,& Andrew G. Parrent , Terry M. Peters , Ali R. Khan Keywords: brain; atlas; human; neuroanatomy; zona incerta; 7T; deep brain stimulation; T1; quantitative MRI 4 5 1Department of Clinical Neurological Sciences, Division of Neurosurgery, Western University, London, Ontario, Canada 6 2Imaging Research Laboratories, Robarts Research Institute Canada, Western University, London, Ontario, Canada 7 3Centre for Functional and Metabolic Mapping, Robarts Research Institute, Western University, London, Ontario, Canada 8 4School of Biomedical Engineering, Western University, London, Ontario, Canada 9 5Brain and Mind Institute, Western University, London, Ontario, Canada 10 6Department of Medical Biophysics, Western University, London, Ontario, Canada 11 7IBS Center for Neuroscience Imaging Research, Sungkyunkwan University, Seobu-ro, 2066, Jangan-gu, Suwon, South Korea 12 8Department of Biomedical Engineering, N Center, Sungkyunkwan University, Seobu-ro, 2066, Jangan-gu, Suwon, South Korea 13 9Techna Institute and Koerner Scientist in MR Imaging, University Health Network, 100 College St, Toronto, ON, Canada 14 *Corresponding author 15 &Joint senior authors 16 17 Abstract: The zona incerta (ZI) is a small gray matter region of the deep brain first identified in the 19th 18 century, yet direct in vivo visualization and characterization has remained elusive. -

Coordination of Rapid Cholinergic and Dopaminergic Signaling in Striatum During Spontaneous Movement
RESEARCH ARTICLE Coordination of rapid cholinergic and dopaminergic signaling in striatum during spontaneous movement Mark Howe†*, Imane Ridouh, Anna Letizia Allegra Mascaro‡, Alyssa Larios, Maite Azcorra, Daniel A Dombeck* Department of Neurobiology, Northwestern University, Evanston, United States Abstract Interplay between dopaminergic and cholinergic neuromodulation in the striatum is crucial for movement control, with prominent models proposing pro-kinetic and anti-kinetic effects of dopamine and acetylcholine release, respectively. However, the natural, movement-related signals of striatum cholinergic neurons and their relationship to simultaneous variations in dopamine signaling are unknown. Here, functional optical recordings in mice were used to establish rapid cholinergic signals in dorsal striatum during spontaneous movements. Bursts across the cholinergic population occurred at transitions between movement states and were marked by *For correspondence: widespread network synchronization which diminished during sustained locomotion. Simultaneous [email protected] (MH); cholinergic and dopaminergic recordings revealed distinct but coordinated sub-second signals, [email protected] suggesting a new model where cholinergic population synchrony signals rapid changes in (DAD) movement states while dopamine signals the drive to enact or sustain those states. DOI: https://doi.org/10.7554/eLife.44903.001 Present address: †Department of Psychological and Brain Sciences, Boston University, Boston, United States; ‡European Laboratory for Non- Introduction linear Spectroscopy, Florence, Striatum cholinergic interneurons (ChIs) have long been recognized as critical for normal functioning Italy and Neuroscience Institute, of the basal ganglia in the control of movement and in learning and responding to motivational and National Research Council, Pisa, salient stimuli (Apicella et al., 1996; Apicella et al., 1997; Apicella et al., 1991; Collins et al., Italy 2016; Kimura et al., 1984; Kondabolu et al., 2016; Schulz et al., 2011; Kaneko et al., 2000). -

Targeted Ablation of Cholinergic Interneurons in the Dorsolateral Striatum Produces Behavioral Manifestations of Tourette Syndrome
Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome Meiyu Xua, Andrew Kobetsa,1, Jung-Chieh Dua,2, Jessica Lenningtonb, Lina Lia, Mounira Banasra, Ronald S. Dumana,c,d, Flora M. Vaccarinob,d,e, Ralph J. DiLeonea,d,e, and Christopher Pittengera,b,d,f,3 Departments of aPsychiatry, cPharmacology, eNeurobiology, fPsychology, and the bChild Study Center and dInterdepartmental Neuroscience Program, Yale University, New Haven, CT 06519 Edited by Floyd Bloom, The Scripps Research Institute, La Jolla, CA, and approved December 15, 2014 (received for review October 10, 2014) Gilles de la Tourette syndrome (TS) is characterized by tics, which with TS, whereas abnormalities in medial networks correlated are transiently worsened by stress, acute administration of dopa- instead with comorbid obsessive-compulsive symptoms (23). minergic drugs, and by subtle deficits in motor coordination and The principal cells of the striatum, the GABAergic medium sensorimotor gating. It represents the most severe end of a spectrum spiny neurons (MSNs), are modulated by several types of in- of tic disorders that, in aggregate, affect ∼5% of the population. terneuron (24). Recent postmortem work has revealed that spe- Available treatments are frequently inadequate, and the patho- cific interneuron populations are abnormal in patients with severe, physiology is poorly understood. Postmortem studies have revealed refractory TS (25–27). Cholinergic interneurons, identified by a reduction in specific striatal interneurons, including the large cho- their expression of choline acetyltransferase (ChAT), are critical linergic interneurons, in severe disease. We tested the hypothesis regulators of striatal function, although they constitute only about that this deficit is sufficient to produce aspects of the phenomenol- 1% of all neurons in the striatum (24). -

Outcomes from Stereotactic Surgery for Essential Tremor
Movement disorders J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp-2018-318240 on 18 October 2018. Downloaded from REVIEW Outcomes from stereotactic surgery for essential tremor Robert Francis Dallapiazza,1 Darrin J Lee,1 Philippe De Vloo,1 Anton Fomenko,1 Clement Hamani,1 Mojgan Hodaie,1 Suneil K Kalia,1 Alfonso Fasano,2,3,4 Andres M Lozano1 ► Additional material is ABSTRact can profoundly impact the quality of life in patients published online only. To view There are several different surgical procedures that with severe symptoms.5 6 Patients report difficulty please visit the journal online with everyday tasks such as eating, drinking, hand- (http:// dx. doi. org/ 10. 1136/ are used to treat essential tremor (ET), including deep jnnp- 2018- 318240). brain stimulation (DBS) and thalamotomy procedures writing and dressing. Many patients also experi- with radiofrequency (RF), radiosurgery (RS) and most ence social embarrassment regarding their tremor. 1Division of Neurosurgery, recently, focused ultrasound (FUS). Choosing a surgical Some will refrain from dining in public, and others University of Toronto, Toronto, treatment requires a careful presentation and discussion will retire from employment early due to disabling Ontario, Canada 2Edmond J. Safra Program in of the benefits and drawbacks of each.W e conducted a symptoms. Parkinson’s Disease, Morton literature review to compare the attributes and make an Medical therapy is the first-line treatment for and Gloria Shulman Movement appraisal of these various procedures. DBS was the most ET (particularly beta-blockers and primidone).7 Disorders Clinic, Toronto commonly reported treatment for ET. One-year tremor However, medications alone are often insufficient Western Hospital, Toronto, reductions ranged from 53% to 63% with unilateral to control severe symptoms and can have undesir- Ontario, Canada 8 3Division of Neurology, Vim DBS. -

Neural Dysfunction Linked to Inositol Polyphosphate Multikinase
Huntington’s disease: Neural dysfunction linked to inositol polyphosphate multikinase Ishrat Ahmeda, Juan I. Sbodioa, Maged M. Harraza, Richa Tyagia, Jonathan C. Grimaa, Lauren K. Albacarysa, Maimon E. Hubbib, Risheng Xua, Seyun Kimc,1, Bindu D. Paula,1,2, and Solomon H. Snydera,d,e,1,2 aThe Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 21205; bMcKusick–Nathans Institute of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205; cDepartment of Biological Sciences, Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea; dDepartment of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205; and eDepartment of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205 Contributed by Solomon H. Snyder, June 18, 2015 (sent for review April 23, 2015) Huntington’s disease (HD) is a progressive neurodegenerative disease for CREB-binding protein (17), p53 (18), and serum response factor caused by a glutamine repeat expansion in mutant huntingtin (mHtt). (19). Despite these insights into the functions of IPMK, its phys- Despite the known genetic cause of HD, the pathophysiology of this iologic and pathologic regulation have been largely unexplored. disease remains to be elucidated. Inositol polyphosphate multikinase We report that IPMK is profoundly depleted in HD, reflecting (IPMK) is an enzyme that displays soluble inositol phosphate kinase the influence of mHtt upon Ctip2, a putative striatal-enriched activity, lipid kinase activity, and various noncatalytic interactions. We transcription factor for IPMK. We show that IPMK depletion report a severe loss of IPMK in the striatum of HD patients and mediates neural dysfunction, because virally mediated restoration in several cellular and animal models of the disease. -
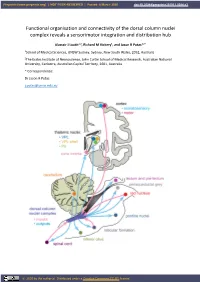
Functional Organisation and Connectivity of the Dorsal Column Nuclei Complex Reveals a Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Functional organisation and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Abstract The dorsal column nuclei complex (DCN-complex) includes the dorsal column nuclei (DCN, referring to the gracile and cuneate nuclei collectively), external cuneate, X, and Z nuclei, and the median accessory nucleus. The DCN are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. To consolidate insights into their sensorimotor functions, this review examines the morphology, organisation, and connectivity of the DCN and their associated nuclei. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the remaining constituents of the DCN-complex.