Apple Thesis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

European Apple and Pear Crop Forecast 2011
EUROPEAN APPLE AND PEAR CROP FORECAST AUGUST 2011 9 10 August 2011 FOREWORD WAPA, the World Apple and Pear Association, is pleased to provide the 2011 European apple and pear crop estimate. This data will be released on the occasion of the 35th Prognosfruit Conference, which will take place in Ljubljana, Slovenia from 4-6 August 2011. This report is compiled upon the initiative of the apple and pear Working Group of COPA COGECA. The data has been collected with the useful support of the respective representative national producer organisations of the various Member States of the European Union and beyond. In regard to 2011, this report concludes that apple production in the EU for the 21 top producing countries contributing to this report will increase by 5% compared to the previous year, corresponding to a production of 10.195.000T. This production is however a 5% lower than the average for the last three years. In regard to varieties, Golden Delicious production will be up by 5% to 2.533.000T. Gala will increase by 7% to 1.059.000T. Jonagold will be up by 14% at 594.000T, while Red Delicious will decrease by 4% to 635.000T. Regarding pears, European growers predict a higher crop by 12% compared to 2010. Indeed, it is reported that the total crop in 2011 will reach 2.533.000T, compared to 2010 production which reached 2.264.000T. This figure relates to the production of the top 18 Member States of the EU-27 growing pears and contributing with their data to this report. -

Ardrossan Sets Sights on New Growth CONFIDENCE GROWS HERE
Australian Fruitgrower Winter 2020 • Vol 14 • Issue 2 Global knowledge at your fingertips SWP redefines ‘unskilled’ Pruning for vigour Ardrossan sets sights on new growth CONFIDENCE GROWS HERE Introducing a new level of confi dence in DMIs New Belanty fungicide gives you a new level of confi dence in the control of black spot in apples. Setting a new global standard for DMI’s, Belanty provides up to 100 times stronger enzyme binding than other DMI’s and is able to control resistant target disease populations. After years of research, Belanty is the breakthrough you’ve been waiting for. Find out more at crop-solutions.basf.com.au ALWAYS READ AND FOLLOW LABEL DIRECTIONS. © Copyright BASF 2020 ® Registered trademark of BASF. W244376 05.2020 CONTENTS A P A L NEWS LABOUR CEO Report . .04 Advocacy update . .05 Global knowledge at your fingertips . .12 21 FEA TURE 06 Seasonal workers redefine ‘unskilled’ Emerging BIOSECURITY stronger Biosecurity – what’s in it for me? . .26 Ardrossan sets sights on new growth . 06 RAISING T H E BAR : Tree returns must justify water cost . .08 R&D - LED INSIGH T S I N T O S M A R TER GROW T H Protect market access . .09 Focus on output . .09 Water security must come first . .10 Identify the opportunities . .11 28 EXPORT Online export training from July . .14 Pruning for vigour S T A T E R O UNDUP management State roundups . .16 MARKETING Campaign adds ‘feel good’ factor . .23 Pears that will arrive well before the heirs . 34 Sundial Orchard: Illuminating the future . -

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

CMI Orchards Expansion a Boon for Retailers
- Advertisement - CMI Orchards expansion a boon for retailers August 12, 2020 Furthering its commitment to being a one-stop-shop for retailers looking for premium apples, pears and cherries grown in Washington state, CMI Orchards LLC reports continued expansion for the company. Strategic partnerships with Yakima Fruit, as well as with Pine Canyon Growers, in addition to significant internal growth, has added over 4 million boxes to CMI’s manifest. “This is all a part of a long-range plan for strategic growth to better serve our customers,” said CMI President Bob Mast. “The design began to unfold back in 2018 when CMI Orchards added Pine Canyon Growers as a grower, packer and shipper. This addition greatly strengthened our manifest and our progress has enabled us to team up with another great partner in Yakima Fruit.” “Yakima Fruit, formerly connected to Yakima Fresh, has a highly desirable manifest that greatly bolsters our offerings, including exceptional early Honeycrisp and high-colored genetic strains of Honeycrisp, Gala and Fuji,” said Mast. “The partnership also adds considerable Cosmic Crisp volume.” The partnership was executed on March 20. As a result of recent growth, CMI reports the following increases in its core, organic and branded offerings: Granny Smith (89 percent), Gala (44 percent), Pink Lady (92 percent), Fuji (62 percent), Honeycrisp (62 percent), and organic Honeycrisp (47 percent). According to CMI, its total organic crop has surpassed 3.5 million boxes, which is made up of increases in core organic varieties such as Gala, Fuji and Honeycrisp, in addition to branded organics, which create opportunities for retail pricing tiers that help boost sales. -

Spring Issue Dog-Friendly Adams County! Tickets on Sale: June 1, 2018 Spring and Fall Are the Seasons with the Most Appropriate Names
MAGAZINE May / June 2018 COMPLIMENTARY Spring Issue Dog-Friendly Adams County! TICKETS ON SALE: JUNE 1, 2018 Spring and fall are the seasons with the most appropriate names. MAGAZINE MAY / JUNE 2018 We spring into brightness. Colorful flowers and delicious fruits and A publication of Gettysburg Times, LLC vegetables spring to life. In PO Box 3669, Gettysburg, PA September, they begin to fall to death. In this issue of Companion, we focus PUBLISHER on the rebirth - spring. Harry Hartman Holly Fletcher and Mary Grace Keller EDITOR help readers focus in on spring goals Alex J. Hayes of planting your own garden and MAGAZINE DESIGN growing your own vegetables. Kristine Celli Jim Hale explains how these BY ALEX J. HAYES wonderful plants are pollinated with CONTRIBUTING our buzzing friends. WRITERS Speaking of friends, two close friends Holly Fletcher of mine - my Golden Retriever Toby Jim Hale and Black Lab Callie - make their Mary Grace Keller Josh Martin Companion debut in a Vanessa A Note Vanessa Pellechio Pellechio feature about dog-friendly From The Gettysburg. PHOTOGRAPHY We round out this issue with John Armstrong another feature by Josh Martin about Holly Fletcher one of Adams County’s Mary Grace Keller all-star athletes. Jim Hale Editor We hope you enjoy this edition Darryl Wheeler and spring breathes new life into ADVERTISING SALES your world. Brooke Asper Tanya Parsons Nancy Pritt What’s Inside: David Kelly The Gettysburg Companion is published bimonthly and distributed throughout CALENDAR ����������������������������������������4 the area. The Gettysburg Companion can be mailed to you for $27 per year (six ����������������������� 7 issues) or $42 for two years (12 issues). -

Branched-Chain Ester Biosynthesis in Ripening Apple Fruit
BRANCHED-CHAIN ESTER BIOSYNTHESIS IN RIPENING APPLE FRUIT By Nobuko Sugimoto A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Horticulture 2011 ABSTRACT BRANCHED-CHAIN ESTER BIOSYNTHESIS IN RIPENING APPLE FRUIT By Nobuko Sugimoto In apple fruit, aroma is an essential element of organoleptic quality and it can suffer in response to a number of pre- and post-harvest cultural treatments. Of the several classes of odor-active compounds, esters are the most important, but little is known regarding pathways of biosynthesis. This research presents evidence for a ‘new’ pathway for ester biosynthesis in apple that uses the starting products pyruvate and acetyl-CoA for the synthesis of precursors to branched-chain (BC) and certain short, straight-chain (SC) esters. The initial step in the pathway involves the formation of citramalic acid from pyruvate and acetyl-CoA by citramalate synthase (CIM). Citramalic acid then provides for the formation of α-keto-β-methylvalerate and its tramsaminated product isoleucine via α-ketobutyrate, and also for the BC ester precursors 2-methylbutanol or 2- methylbutanoate. The hypothesized pathway also provides for the formation of 3-, 4-, and 5- carbon fatty acids via the process of single-carbon elongation of α-keto acids, which are metabolized to short-chain fatty acids. These short-chain fatty acids are proposed to contribute to SC ester formation. Analysis of ripening fruit revealed that citramalic acid increased about 120- fold as ester production increased during ripening. At the same time, the content of isoleucine increased more than 20-fold, while other amino acids remained steady or declined. -
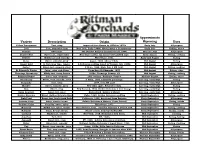
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

Bristol Naturalist News
Contents / Diary of events NOVEMBER 2017 Bristol Naturalist News Discover Your Natural World Bristol Naturalists’ Society BULLETIN NO. 565 NOVEMBER 2017 BULLETIN NO. 565 NOVEMBER 2017 Bristol Naturalists’ Society Discover Your Natural World Registered Charity No: 235494 www.bristolnats.org.uk HON. PRESIDENT: Andrew Radford, Professor CONTENTS of Behavioural Ecology, Bristol University 3 Diary of Events ACTING CHAIRMAN: Stephen Fay HON. PROCEEDINGS RECEIVING EDITOR: 4 Society Walk / Society Talk Dee Holladay, 15 Lower Linden Rd., Clevedon, 5 Lesley’s “Natty News…” BS21 7SU [email protected] HON. SEC.: Lesley Cox 07786 437 528 6 Get Published! Write for Nature in Avon [email protected] HON. MEMBERSHIP SEC: Mrs. Margaret Fay 7 Joint BNS/University programme 81 Cumberland Rd., BS1 6UG. 0117 921 4280 8 Phenology ; Book Club [email protected] Welcome to new members HON. TREASURER: Michael Butterfield 14 Southdown Road, Bristol, BS9 3NL 9 Society Walk Report; (0117) 909 2503 [email protected] Poem for the month BULLETIN DISTRIBUTION 10 BOTANY SECTION Hand deliveries save about £800 a year, so help Botanical notes; Meeting Report; is much appreciated. Offers please to: Plant Records HON. CIRCULATION SEC.: Brian Frost, 60 Purdy Court, New Station Rd, Fishponds, Bristol, BS16 13 GEOLOGY SECTION 3RT. 0117 9651242. [email protected] He will be pleased to supply further details. Also 14 INVERTEBRATE SECTION Notes for this month contact him about problems with (non-)delivery. BULLETIN COPY DEADLINE: 7th of month before 15 LIBRARY Hand-coloured books publication to the editor: David B Davies, The Summer House, 51a Dial Hill Rd., Clevedon, 17 ORNITHOLOGY SECTION BS21 7EW. -

Cedar-Apple Rust
DIVISION OF AGRICULTURE RESEARCH & EXTENSION Agriculture and Natural Resources University of Arkansas System FSA7538 Cedar-Apple Rust Stephen Vann Introduction Assistant Professor One of the most spectacular Extension Urban Plant Pathologist diseases to appear in spring is cedar- apple rust. This disease is caused by the fungus Gymnosporangium juniperi-virginianae and requires both cedar and apple trees to survive each year. It is mainly a problem in the eastern portion of North America and is most important on apple or crab Figure 2. Cedar-apple rust on crabapple apple (Malus sp), but can also affect foliage. quince and hawthorn. yellow-orange color (Figures 1 and 2). Symptoms On the upper leaf surface of these spots, the fungus produces specialized The chief damage by this disease fruiting bodies called spermagonia. On occurs on apple trees, causing early the lower leaf surface (and sometimes leaf drop and poor quality fruit. This on fruit), raised hair-like fruiting bod can be a significant problem to com ies called aecia (Figure 3) appear as mercial apple growers but also harms microscopic cup-shaped structures. the appearance of ornamental crab Wet, rainy weather conditions favor apples in the home landscape. On severe infection of the apple. The apple, symptoms first appear as fungus forms large galls on cedar trees small green-yellow leaf or fruit spots in the spring (see next section), but that gradually enlarge to become a these structures do not greatly harm Arkansas Is Our Campus Visit our web site at: Figure 1. Cedar-apple rust (leaf spot) on Figure 3. Aecia of cedar-apple rust on https://www.uaex.uada.edu apple (courtesy J. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion