Gene Section Review
Total Page:16
File Type:pdf, Size:1020Kb
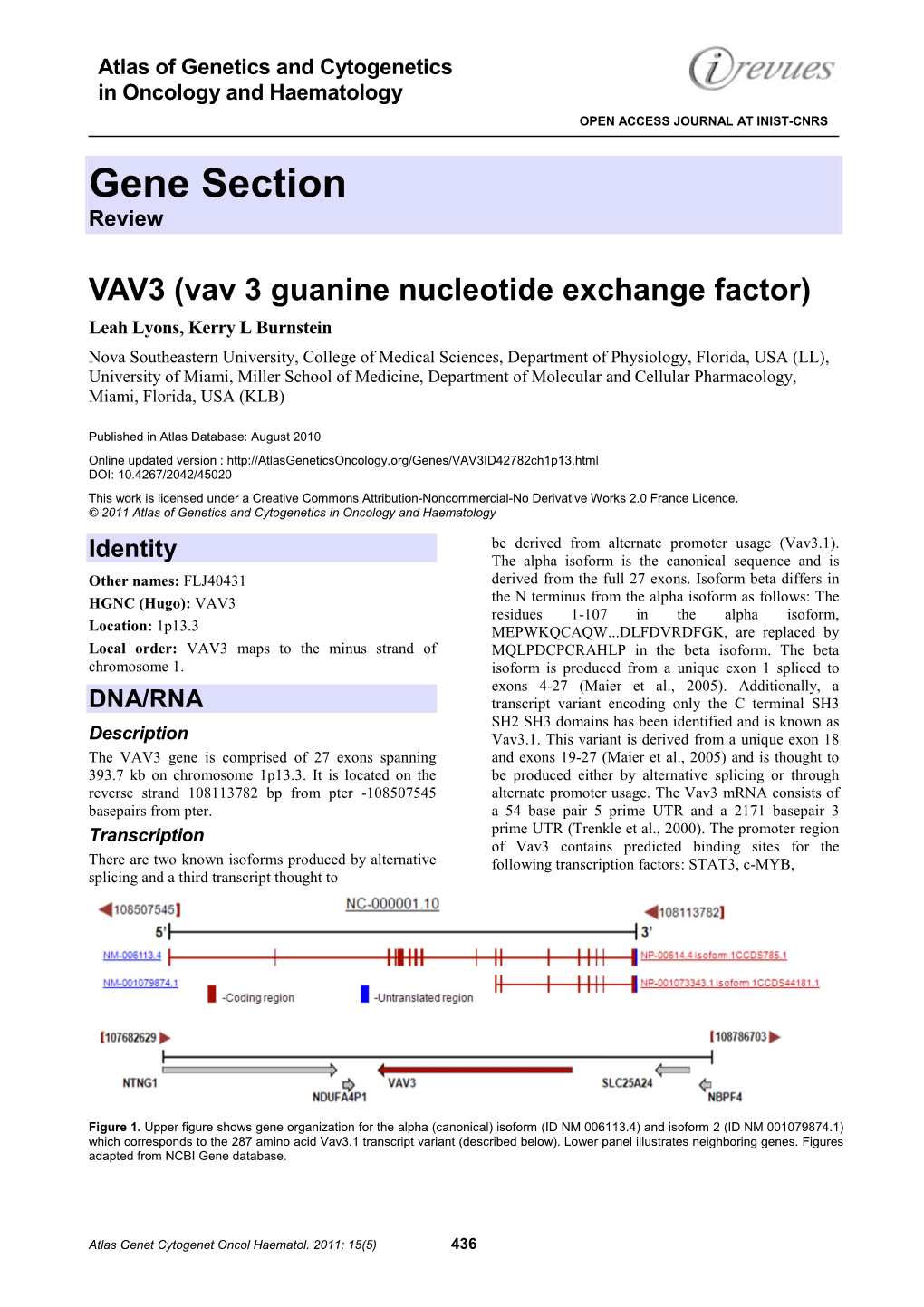
Load more
Recommended publications
-

Anti-VAV3 (Aa 567-578) Polyclonal Antibody (DPABH-12442) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-VAV3 (aa 567-578) polyclonal antibody (DPABH-12442) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Antigen Description Exchange factor for GTP-binding proteins RhoA, RhoG and, to a lesser extent, Rac1. Binds physically to the nucleotide-free states of those GTPases. Plays an important role in angiogenesis. Its recruitement by phosphorylated EPHA2 is critical for EFNA1-induced RAC1 GTPase activation and vascular endothelial cell migration and assembly. Immunogen Synthetic peptide: CSGEQGTLKLPEK, corresponding to internal sequence amino acids 567-578 of Human VAV3 Isotype IgG Source/Host Goat Species Reactivity Human Purification Immunogen affinity purified Conjugate Unconjugated Applications WB, IHC-P Format Liquid Size 50 μg Buffer pH: 7.40; Constituents: 0.5% BSA, Tris buffered saline Preservative 0.02% Sodium Azide Storage Store at 4°C or at -20°C for long term storage. GENE INFORMATION Gene Name VAV3 vav 3 guanine nucleotide exchange factor [ Homo sapiens ] Official Symbol VAV3 Synonyms VAV3; vav 3 guanine nucleotide exchange factor; vav 3 oncogene; guanine nucleotide exchange factor VAV3; VAV-3; FLJ40431; 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics All Rights Reserved Entrez Gene ID 10451 Protein Refseq NP_001073343 UniProt ID Q9UKW4 Chromosome Location 1p13.3 Pathway B cell receptor signaling pathway; Cell death signalling via NRAGE, NRIF and NADE; Chemokine signaling pathway; Coregulation of Androgen receptor activity; EGFR1 Signaling Pathway; Function GTPase activator activity; Rac guanyl-nucleotide exchange factor activity; SH3/SH2 adaptor activity; epidermal growth factor receptor binding; guanyl-nucleotide exchange factor activity; metal ion binding; protein binding 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 2 © Creative Diagnostics All Rights Reserved. -

Chromosome Abnormalities in Two Patients with Features of Autosomal Dominant Robinow Syndrome
ß 2007 Wiley-Liss, Inc. American Journal of Medical Genetics Part A 143A:1790–1795 (2007) Research Letter Chromosome Abnormalities in Two Patients With Features of Autosomal Dominant Robinow Syndrome Juliana F. Mazzeu,1 Ana Cristina Krepischi-Santos,1 Carla Rosenberg,1 Karoly Szuhai,2 Jeroen Knijnenburg,2 Janneke M.M. Weiss,3 Irina Kerkis,1 Zan Mustacchi,4 Guilherme Colin,5 Roˆmulo Mombach,6 Rita de Ca´ssia M. Pavanello,1 Paulo A. Otto,1 and Angela M. Vianna-Morgante1* 1Centro de Estudos do Genoma Humano, Departamento de Gene´tica e Biologia Evolutiva, Instituto de Biocieˆncias, Universidade de Sa˜o Paulo, Sa˜o Paulo, Brazil 2Department of Molecular Cell Biology, Leiden University Medical Center, Leiden, The Netherlands 3Department of Clinical Genetics, Leiden University Medical Center, Leiden, The Netherlands 4Hospital Infantil Darcy Vargas, Sa˜o Paulo, Brazil 5Departamento de Gene´tica Me´dica, Univille, Joinville, Brazil 6Centrinho Prefeito Luiz Gomes, Secretaria Municipal de Sau´de, Joinville, Brazil Received 13 April 2006; Accepted 13 December 2006 How to cite this article: Mazzeu JF, Krepischi-Santos AC, Rosenberg C, Szuhai K, Knijnenburg J, Weiss JMM, Kerkis I, Mustacchi Z, Colin G, Mombach R, Pavanello RM, Otto PA, Vianna-Morgante AM. 2007. Chromosome abnormalities in two patients with features of autosomal dominant Robinow syndrome. Am J Med Genet Part A 143A:1790–1795. To the Editor: Patient 1 Robinow syndrome [OMIM 180700] is characteriz- At age 3 4/12 years the girl was diagnosed as ed by fetal facies, mesomelic dwarfism, and hypo- affected by DRS (Fig. 1A). Detailed clinical examina- plastic genitalia. -

Vav1 (D45G3) Rabbit Mab A
Revision 1 C 0 2 - t Vav1 (D45G3) Rabbit mAb a e r o t S Orders: 877-616-CELL (2355) [email protected] Support: 877-678-TECH (8324) 7 5 Web: [email protected] 6 www.cellsignal.com 4 # 3 Trask Lane Danvers Massachusetts 01923 USA For Research Use Only. Not For Use In Diagnostic Procedures. Applications: Reactivity: Sensitivity: MW (kDa): Source/Isotype: UniProt ID: Entrez-Gene Id: WB, IP H Endogenous 95 Rabbit IgG P15498 7409 Product Usage Information Application Dilution Western Blotting 1:1000 Immunoprecipitation 1:50 Storage Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody. Specificity / Sensitivity Vav1 (D45G3) Rabbit mAb recognizes endogenous levels of total Vav1 protein. Species Reactivity: Human Species predicted to react based on 100% sequence homology: Monkey Source / Purification Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to residues in the carboxy terminus of human Vav1 protein. Background Vav proteins belong to the Dbl family of guanine nucleotide exchange factors (GEFs) for Rho/Rac small GTPases. The three identified mammalian Vav proteins (Vav1, Vav2 and Vav3) differ in their expression. Vav1 is expressed only in hematopoietic cells and is involved in the formation of the immune synapse. Vav2 and Vav3 are more ubiquitously expressed. Vav proteins contain the Dbl homology domain, which confers GEF activity, as well as protein interaction domains that allow them to function in pathways regulating actin cytoskeleton organization (reviewed in 1). -

BRG1 Knockdown Inhibits Proliferation Through Multiple Cellular Pathways in Prostate Cancer Katherine A
Giles et al. Clin Epigenet (2021) 13:37 https://doi.org/10.1186/s13148-021-01023-7 RESEARCH Open Access BRG1 knockdown inhibits proliferation through multiple cellular pathways in prostate cancer Katherine A. Giles1,2,3, Cathryn M. Gould1, Joanna Achinger‑Kawecka1,4, Scott G. Page2, Georgia R. Kafer2, Samuel Rogers2, Phuc‑Loi Luu1,4, Anthony J. Cesare2, Susan J. Clark1,4† and Phillippa C. Taberlay3*† Abstract Background: BRG1 (encoded by SMARCA4) is a catalytic component of the SWI/SNF chromatin remodelling com‑ plex, with key roles in modulating DNA accessibility. Dysregulation of BRG1 is observed, but functionally uncharacter‑ ised, in a wide range of malignancies. We have probed the functions of BRG1 on a background of prostate cancer to investigate how BRG1 controls gene expression programmes and cancer cell behaviour. Results: Our investigation of SMARCA4 revealed that BRG1 is over‑expressed in the majority of the 486 tumours from The Cancer Genome Atlas prostate cohort, as well as in a complementary panel of 21 prostate cell lines. Next, we utilised a temporal model of BRG1 depletion to investigate the molecular efects on global transcription programmes. Depleting BRG1 had no impact on alternative splicing and conferred only modest efect on global expression. How‑ ever, of the transcriptional changes that occurred, most manifested as down‑regulated expression. Deeper examina‑ tion found the common thread linking down‑regulated genes was involvement in proliferation, including several known to increase prostate cancer proliferation (KLK2, PCAT1 and VAV3). Interestingly, the promoters of genes driving proliferation were bound by BRG1 as well as the transcription factors, AR and FOXA1. -

The Human Gene Connectome As a Map of Short Cuts for Morbid Allele Discovery
The human gene connectome as a map of short cuts for morbid allele discovery Yuval Itana,1, Shen-Ying Zhanga,b, Guillaume Vogta,b, Avinash Abhyankara, Melina Hermana, Patrick Nitschkec, Dror Friedd, Lluis Quintana-Murcie, Laurent Abela,b, and Jean-Laurent Casanovaa,b,f aSt. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY 10065; bLaboratory of Human Genetics of Infectious Diseases, Necker Branch, Paris Descartes University, Institut National de la Santé et de la Recherche Médicale U980, Necker Medical School, 75015 Paris, France; cPlateforme Bioinformatique, Université Paris Descartes, 75116 Paris, France; dDepartment of Computer Science, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; eUnit of Human Evolutionary Genetics, Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Institut Pasteur, F-75015 Paris, France; and fPediatric Immunology-Hematology Unit, Necker Hospital for Sick Children, 75015 Paris, France Edited* by Bruce Beutler, University of Texas Southwestern Medical Center, Dallas, TX, and approved February 15, 2013 (received for review October 19, 2012) High-throughput genomic data reveal thousands of gene variants to detect a single mutated gene, with the other polymorphic genes per patient, and it is often difficult to determine which of these being of less interest. This goes some way to explaining why, variants underlies disease in a given individual. However, at the despite the abundance of NGS data, the discovery of disease- population level, there may be some degree of phenotypic homo- causing alleles from such data remains somewhat limited. geneity, with alterations of specific physiological pathways under- We developed the human gene connectome (HGC) to over- come this problem. -

Functional Consequences of Somatic Mutations in Cancer Using Protein
Vuong et al. Genome Medicine 2014, 6:81 http://genomemedicine.com/content/6/10/81 RESEARCH Open Access Functional consequences of somatic mutations in cancer using protein pocket-based prioritization approach Huy Vuong1†, Feixiong Cheng1†, Chen-Ching Lin1 and Zhongming Zhao1,2,3,4* Abstract Background: Recently, a number of large-scale cancer genome sequencing projects have generated a large volume of somatic mutations; however, identifying the functional consequences and roles of somatic mutations in tumorigenesis remains a major challenge. Researchers have identified that protein pocket regions play critical roles in the interaction of proteins with small molecules, enzymes, and nucleic acid. As such, investigating the features of somatic mutations in protein pocket regions provides a promising approach to identifying new genotype-phenotype relationships in cancer. Methods: In this study, we developed a protein pocket-based computational approach to uncover the functional consequences of somatic mutations in cancer. We mapped 1.2 million somatic mutations across 36 cancer types from the COSMIC database and The Cancer Genome Atlas (TCGA) onto the protein pocket regions of over 5,000 protein three-dimensional structures. We further integrated cancer cell line mutation profiles and drug pharmacological data from the Cancer Cell Line Encyclopedia (CCLE) onto protein pocket regions in order to identify putative biomarkers for anticancer drug responses. Results: We found that genes harboring protein pocket somatic mutations were significantly enriched in cancer driver genes. Furthermore, genes harboring pocket somatic mutations tended to be highly co-expressed in a co-expressed protein interaction network. Using a statistical framework, we identified four putative cancer genes (RWDD1, NCF1, PLEK, and VAV3), whose expression profiles were associated with overall poor survival rates in melanoma, lung, or colorectal cancer patients. -

The Molecular Mechanism of Vav3 Oncogene on Upregulation of Androgen Receptor Activity in Prostate Cancer Cells
623-633 20/1/2010 01:24 ÌÌ Page 623 INTERNATIONAL JOURNAL OF ONCOLOGY 36: 623-633, 2010 The molecular mechanism of Vav3 oncogene on upregulation of androgen receptor activity in prostate cancer cells YIN LIU1, XIAOYANG WU1, ZHONGYUN DONG2 and SHAN LU1 Departments of 1Pathology and 2Medicine, University of Cincinnati College of Medicine, the Metabolic Diseases Institute, Cincinnati, OH 45237, USA Received October 15, 2009; Accepted December 2, 2009 DOI: 10.3892/ijo_00000538 Abstract. Our previous studies revealed that Vav3 oncogene primarily expressed in hematopoietic cells, while Vav2 and is overexpressed in human prostate cancer, enhances Vav3 are more ubiquitously expressed. Vav proteins contain androgen receptor (AR)-mediated signaling, and may play a multiple function motifs and are involved in various cellular role in prostate cancer development and progression. The signaling processes, including cytoskeleton organization, purpose of this study was to determine the molecular mecha- calcium influx, phagocytosis and cell transformation (3). Vav nisms responsible for AR activation by Vav3. We found that proteins share a common structure, including a N-terminal interaction between N-terminus and C-terminus of AR is calponin homology (CH) domain involved in Ca+2 mobilization essential for its elevated activity stimulated by Vav3. The DH and transforming activity, an acidic domain (AD) containing and PH domains of Vav3 are involved in direct interaction three regulatory tyrosines, a Dbl homology (DH) domain with with AR. Both cytoplasmic and nuclear levels of AR and a conserved region that promotes the exchange of GDP for Vav3 are elevated and their nuclear localization is further GTP on Rac/Rho GTPases, a pleckstrin homology (PH) stimulated by DHT in androgen-independent LNCaP-AI cells domain binding to PIP3 that enables its movement to the relative to their parental androgen-dependent LNCaP cells. -

Guanine Nucleotide Exchange Factors for Rho Gtpases: Turning on the Switch
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Guanine nucleotide exchange factors for Rho GTPases: turning on the switch Anja Schmidt1,3 and Alan Hall1,2 1MRC Laboratory for Molecular Cell Biology, Cancer Research UK Oncogene and Signal Transduction Group, and 2Department of Biochemistry and Molecular Biology, University College London, London WC1E 6BT, UK Rho GTPases control many aspects of cell behavior Structural features through the regulation of multiple signal transduction The first mammalian GEF, Dbl, isolated in 1985 as an pathways (Van Aelst and D’Souza-Schorey 1997; Hall oncogene in an NIH 3T3 focus formation assay using 1998). Rho, Rac, and Cdc42were first recognized in the DNA from a human diffuse B-cell lymphoma (Eva and early 1990s for their unique ability to induce specific ∼ filamentous actin structures in fibroblasts; stress fibers, Aaronson 1985), was found to contain a region of 180 lamellipodia/membrane ruffles, and filopodia, respec- amino acids that showed significant sequence similarity tively (Hall 1998). Over the intervening years, evidence to CDC24, a protein identified genetically as an up- has accumulated to show that in all eukaryotic cells, stream activator of CDC42in yeast (Bender and Pringle Rho GTPases are involved in most, if not all, actin-de- 1989; Ron et al. 1991). Dbl was subsequently shown to pendent processes such as those involved in migration, catalyze nucleotide exchange on human Cdc42in vitro adhesion, morphogenesis, axon guidance, and phagocy- (Hart et al. 1991), and a conserved domain in Dbl and tosis (Kaibuchi et al. 1999; Chimini and Chavrier 2000; CDC24, now known as the DH (Dbl homology) domain, Luo 2000). -

A Controlled Vocabulary for Pathway Entities and Events Steve Jupe1,*, Bijay Jassal2, Mark Williams1 and Guanming Wu2
Database, 2014, 1–9 doi: 10.1093/database/bau060 Original article Original article Downloaded from https://academic.oup.com/database/article/doi/10.1093/database/bau060/2634538 by guest on 30 September 2021 A controlled vocabulary for pathway entities and events Steve Jupe1,*, Bijay Jassal2, Mark Williams1 and Guanming Wu2 1European Bioinformatics Institute (EMBL-EBI), European Molecular Biology Laboratory, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SD, UK Downloaded from 2Ontario Institute for Cancer Research, Toronto, ON M5G0A3, Canada *Corresponding author: Tel: þ44 (0)1223 492673; Fax: þ44 (0)1223 492621; Email: [email protected] Citation details: Jupe,S., Jassal,B., Williams,M., Wu,G. A controlled vocabulary for pathway entities and events. Database (2014) Vol. 2014: article ID bau060; doi:10.1093/database/bau060 http://database.oxfordjournals.org/ Received 18 February 2014; Revised 30 April 2014; Accepted 28 May 2014 Abstract Entities involved in pathways and the events they participate in require descriptive and unambiguous names that are often not available in the literature or elsewhere. Reactome is a manually curated open-source resource of human pathways. It is accessible via a website, available as downloads in standard reusable formats and via Representational by guest on November 22, 2016 State Transfer (REST)-ful and Simple Object Access Protocol (SOAP) application pro- gramming interfaces (APIs). We have devised a controlled vocabulary (CV) that creates concise, unambiguous and unique names for reactions (pathway events) and all the mo- lecular entities they involve. The CV could be reapplied in any situation where names are used for pathway entities and events. Adoption of this CV would significantly improve naming consistency and readability, with consequent benefits for searching and data mining within and between databases. -

Novel Recognition Mode Between Vav and Grb2 SH3 Domains
The EMBO Journal Vol. 20 No. 12 pp. 2995±3007, 2001 Novel recognition mode between Vav and Grb2 SH3 domains Motohiko Nishida1,2, Koji Nagata3, SH3 domain, the peptide must adopt a polyproline-type II Yukiko Hachimori3, Masataka Horiuchi1, (PPII) helical conformation. The most famous example of Kenji Ogura1, Valsan Mandiyan4, this recognition is the interaction of growth factor 4 receptor-bound protein 2 (Grb2) with son of sevenless Joseph Schlessinger and (Sos), the guanine nucleotide exchange factor for Ras. 1,2,5 Fuyuhiko Inagaki Grb2 is an adaptor protein consisting of one SH2 domain 1Department of Structural Biology, Graduate School of Pharmaceutical ¯anked by two SH3 domains. It has been well established Sciences, Hokkaido University, N-12, W-6, Kita-ku, Sapporo 060-0812, that Grb2 transmits the upstream signal through its 2CREST, Japan Science and Technology Corporation, association with proline-rich regions of Sos, via the SH3 Motomachi 4-1-8, Kawaguchi 332-0012, 3Department of Molecular Physiology, Tokyo Metropolitan Institute of Medical Science, domains (Egan et al., 1993). As other targets of the Grb2 Honkomagome 3-18-22, Bunkyo-ku, Tokyo 113-861, Japan and SH3 domains, proline-rich proteins such as Cbl, dynamin 4Department of Pharmacology, New York University Medical School, and WASP have been reported (reviewed in Buday, 1999). New York, NY 10016, USA Vav is among the proposed targets of Grb2 (Machide et al., 5Corresponding author 1995; Hanazono et al., 1996; Miyakawa et al., 1997). e-mail: ®[email protected] Vav is a protein with a molecular mass of 95 kDa, which is expressed exclusively in hematopoietic cells (Katzav Vav is a guanine nucleotide exchange factor for the et al., 1989). -

Mouse Vav3 Conditional Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Vav3 Conditional Knockout Project (CRISPR/Cas9) Objective: To create a Vav3 conditional knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Vav3 gene (NCBI Reference Sequence: NM_020505 ; Ensembl: ENSMUSG00000033721 ) is located on Mouse chromosome 3. 27 exons are identified, with the ATG start codon in exon 1 and the TAA stop codon in exon 27 (Transcript: ENSMUST00000046864). Exon 3~4 will be selected as conditional knockout region (cKO region). Deletion of this region should result in the loss of function of the Mouse Vav3 gene. To engineer the targeting vector, homologous arms and cKO region will be generated by PCR using BAC clone RP23-79G21 as template. Cas9, gRNA and targeting vector will be co-injected into fertilized eggs for cKO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Homozygous mutation of this gene results in tachycardia, systemic arterial hypertension, cardiovascular remodeling, hyperactivity of sympathetic neurons and thus high catecholamine levels, and increased levels of kidney- related hormones. Exon 3 starts from about 12.67% of the coding region. The knockout of Exon 3~4 will result in frameshift of the gene. The size of intron 2 for 5'-loxP site insertion: 70670 bp, and the size of intron 4 for 3'-loxP site insertion: 4424 bp. The size of effective cKO region: ~2676 bp. The cKO region does not have any other known gene. Page 1 of 7 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele 5' gRNA region gRNA region 3' 1 3 4 27 Targeting vector Targeted allele Constitutive KO allele (After Cre recombination) Legends Exon of mouse Vav3 Homology arm cKO region loxP site Page 2 of 7 https://www.alphaknockout.com Overview of the Dot Plot Window size: 10 bp Forward Reverse Complement Sequence 12 Note: The sequence of homologous arms and cKO region is aligned with itself to determine if there are tandem repeats. -

Lysine Acetylation Reshapes the Downstream Signaling Landscape of Vav1 in Lymphocytes
cells Article Lysine Acetylation Reshapes the Downstream Signaling Landscape of Vav1 in Lymphocytes Sonia Rodríguez-Fdez 1,2,3 , Lucía Fernández-Nevado 1,2, L. Francisco Lorenzo-Martín 1,2,3 and Xosé R. Bustelo 1,2,3,* 1 Centro de Investigación del Cáncer, CSIC-University of Salamanca, 37007 Salamanca, Spain; [email protected] (S.R.-F.); [email protected] (L.F.-N.); [email protected] (L.F.L.-M.) 2 Instituto de Biología Molecular y Celular del Cáncer, CSIC-University of Salamanca, 37007 Salamanca, Spain 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), CSIC-University of Salamanca, 37007 Salamanca, Spain * Correspondence: [email protected]; Tel.: +34-663194634 Received: 13 January 2020; Accepted: 2 March 2020; Published: 4 March 2020 Abstract: Vav1 works both as a catalytic Rho GTPase activator and an adaptor molecule. These functions, which are critical for T cell development and antigenic responses, are tyrosine phosphorylation-dependent. However, it is not known whether other posttranslational modifications can contribute to the regulation of the biological activity of this protein. Here, we show that Vav1 becomes acetylated on lysine residues in a stimulation- and SH2 domain-dependent manner. Using a collection of both acetylation- and deacetylation-mimicking mutants, we show that the acetylation of four lysine residues (Lys222, Lys252, Lys587, and Lys716) leads to the downmodulation of the adaptor function of Vav1 that triggers the stimulation of the nuclear factor of activated T cells (NFAT). These sites belong to two functional subclasses according to mechanistic criteria. We have also unveiled additional acetylation sites potentially involved in either the stimulation (Lys782) or the downmodulation (Lys335, Lys374) of specific Vav1-dependent downstream responses.