Adsorption of Methylene Blue Dye from Aqueous Solution Using Hyperbranched Polyester: Isotherm and Thermodynamic Studies
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Parliamentary Bulletin
RAJYA SABHA Parliamentary Bulletin PART-II Nos.:54544-54545] THURSDAY, AUGUST 27, 2015 No.54544 Committee Section (Subordinate Legislation) Statutory Orders laid on the Table of the Rajya Sabha during the week ending August 7, 2015 (236th Session) The following Statutory Rules and Orders made under the delegated powers of legislation and published in the Gazette were laid on the Table of the Rajya Sabha during the week ending August 7, 2015. The Orders will be laid on the Table for a period of 30 days, which may be comprised in one session or in two or more successive sessions. Members can move a motion for modification/annulment before the expiry of the session, immediately, following the session in which the laying period of 30 days is completed. 2 Sl. Number and date Brief Subject Date on Provision of the Statute No. of Rule/Order which laid under which laid 1 2 3 4 5 MINISTRY OF AYUSH 1 No. 7-1/2004- The Homoeopathy Central Council 04.08.2015 Section 33 (2) of the CCH(Pt), dated the (Registration) (Amendment) Homoeopathy Central 11th April, 2015. Regulations, 2015 along with delay Council Act, 1973. statement. 2 No. G.S.R. 390 The Drugs and Cosmetics -do- Section 38 of the Drugs and (E), dated the (4th Amendment) Rules, 2015 along Cosmetics Act, 1940. 18th May, 2015. with delay statement. MINISTRY OF COMMERCE AND INDUSTRY (DEPARTMENT OF COMMERCE) 3 No. Admin-1/Estt/ Amending Notification No. Admin- 05.08.2015 Section 40 of the Spices SPEDA/2014, the 1/Estt/SPEDA/ 2014, dated the Board Act, 1986. -

Mahanadi River Basin
The Forum and Its Work The Forum (Forum for Policy Dialogue on Water Conflicts in India) is a dynamic initiative of individuals and institutions that has been in existence for the last ten years. Initiated by a handful of organisations that had come together to document conflicts and supported by World Wide Fund for Nature (WWF), it has now more than 250 individuals and organisations attached to it. The Forum has completed two phases of its work, the first centring on documentation, which also saw the publication of ‘Water Conflicts in MAHANADI RIVER BASIN India: A Million Revolts in the Making’, and a second phase where conflict documentation, conflict resolution and prevention were the core activities. Presently, the Forum is in its third phase where the emphasis of on backstopping conflict resolution. Apart from the core activities like documentation, capacity building, dissemination and outreach, the Forum would be intensively involved in A Situation Analysis right to water and sanitation, agriculture and industrial water use, environmental flows in the context of river basin management and groundwater as part of its thematic work. The Right to water and sanitation component is funded by WaterAid India. Arghyam Trust, Bangalore, which also funded the second phase, continues its funding for the Forums work in its third phase. The Forum’s Vision The Forum believes that it is important to safeguard ecology and environment in general and water resources in particular while ensuring that the poor and the disadvantaged population in our country is assured of the water it needs for its basic living and livelihood needs. -

NSS October to December 2017 Vol. 2
Naveen Shodh Sansar (An International Refereed/ Peer Review Research Journal) (U.G.C. Jr. No. 64728) 1 ISSN 2320-8767, E- ISSN 2394-3793, Impact Factor - 4.710 (2016) October to December 2017, Vol. II Index/AZwH´$_oUH$m 01. Index/ AZwH´$_oUH$m ................................................................................................................ 01 02. Regional Editor Board / Editorial Advisory Board ............................................................... 06/07 03. Referee Board ........................................................................................................................................ 08 04. Spokesperson ............................................................................................................... 10/11 (Science / ndkmZ) 05. Reduction in consumption of limestone by use of Portland Pozzolana Cement Using .................. 12 Mathematical Modeling (Dr. Sapna Shrimali, R.K. Mishra) 06. Common Fixed Point Theorems for in fuzzy metric spaces for Two mapping (Dr. Sangeeta Biley) .. 18 07. A Critical Analysis Of Perception Towards Semester System In Madhya Pradesh, India ................ 20 (Dr. Mamta Prajapati, Dr. Balram Pd. Prajapati) 08. Comparative Phytochemical Studies Of Ocimum Sanctum And Mentha Arvensis Ethanolic ......... 24 Leaf Extract (Dr. Prakash Solanki, Dr. Pramod Pandit, Anil Badore) 09. The Chemistry Of Cobalt In The Soil (Dr. S. K. Udaipure) ................................................................... 27 10. Comparative Study Of Sirpur And Piplyapala Talab -

Water Privatization in Developing Countries: Principles, Implementations and Socio-Economic Consequences
Available online at www.worldscientificnews.com WSN 10 (2015) 17-31 EISSN 2392-2192 Water privatization in developing countries: Principles, implementations and socio-economic consequences Sayan Bhattacharya1,*, Ayantika Banerjee2 1Department of Environmental Studies, Rabindra Bharati University, Kolkata, India 2Department of Environmental science, Asutosh College, Kolkata, India *E-mail address: [email protected] , [email protected] ABSTRACT Water related problems are continuously affecting the social infrastructures and jeopardizing the productivity of modern globalized society. As the water crisis intensifies, several governments around the world are advocating a radical solution: the privatization, commoditization and mass diversion of water. Water privatization involves transferring of water resources control and/or water management services to private companies. The water management service may include operation and management, bill collection, treatment, distribution of water and waste water treatment in a community. The privatization of water has already happened in several developed countries and is being pushed in many developing countries through structural adjustment policies. Water privatization will invariably increase the price of this common property resource because there are hidden costs involved in water collection, purification and distribution systems. Increase in water consumption will be satisfied through the market dynamics often at the cost of the poor who cannot afford the increased water tariffs. The corporations will recover their costs by exploiting the consumers irrespective of their economic conditions. Another possible threat of water privatization is the unsustainable water extraction by the water corporations for maximizing profits and subsequent destructions of water bodies and aquifers. Corporations in search of profits can compromise on water quality in order to reduce costs. -

Dams, Rivers & People
Dams, Rivers & People UPDATE ON RELATED ISSUES SANDRP ISSUE ONE FEBRUARY 2003 INDEX About DRP 1 Irrigation Options 25 River Link: Some Basic Information 2 Hudco money sunk in Irrigation Bonds26 Govt Com Sceptical of River Linking 3 A debate on PIM 28 Proposed East Flowing River Links 4 Groundwater: Rajasthan, Haryana 29 Scepticism about River Link Rhetoric 5 You are Wrong, Mr Prime Minister 30 TU against River Link proposals 6 Publication Available with SANDRP 31 Decommissioning of Dumbur 7 Water Privatisation in Parliament 32 Indictment of Khuga Project by CAG 8 Privatisation Divorced from reality 33 NHPC indicted in CAG Report (2002) 8 QUOTES 34 Kathmandu Declaration 9 Ganga (in)Action Plan 35 Deteriorating Indus Delta 11 Food Management 36 CRBIP Complaint to ADB insp. Panel 12 2002: “All India Drought Year” 37 Drought, State and Civil society 13 Farmgate: Impact of North on South 38 Development as if democracy is real 15 Sugar Scam in Maharashtra 39 Opposition to Bhavani Diversion 16 Small Hydro in Uttaranchal, Nepal 40 News from Narmada valley 17 Power Games of Planners 41 Illegal Public Hearing at Teesta Dam 19 Power Finance News 42 Chamera Coffer dam Washed away 20 President’s Address to Parliament 43 Opposition to Tipaimukh Dam 20 Your Responses 44 Indonesian Dam Affected Sue Japan 23 Politics at WSSD 24 ABOUT DAMS, RIVERS & PEOPLE The DRP will be available both in electronic (text and word To clearly reflect the issues we are dealing with, we have versions) and printed versions. The softcopies of DRP are decided to change the name of our periodical to Dams, also available at www.narmada.org/sandrp and Rivers & People. -
Published by Sampan Media Pvt. Ltd. for Chhattisgarh Tourism Board All Rights Reserved
Published by Sampan Media Pvt. Ltd. for Chhattisgarh Tourism Board All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without the prior permission of the copyright owner. Details like telephone, fax numbers, opening hours, prices and travel information may change. The publisher is not responsible for error, if any. Copyright©2014 Chhattisgarh Tourism Board Published for Chhattisgarh Tourism Board by Sampan Media Pvt. Ltd. Regd. Office: 124, 3rd Floor, Shahpur Jat, Opp. Siri Fort Auditorium, New Delhi – 110049, Ph: 9560264447, [email protected] Contents Introducing Chhattisgarh 4 The Land and its History 5 Tribal Culture 16 Handicrafts 28 Cultural Traditions 39 Destinations 47 Raipur and Around 48 Bilaspur and Around 77 Bastar Division 104 National Parks and Sanctuaries 121 Practical Information 130 Introducing Chhattisgarh Chitrakote Fall The Land and its History hhattisgarh was a part of the state of Madhya Pradesh up till C2000, after which it was declared as a separate state with Raipur as the capital city. The state borders shares the states of Madhya Pradesh, Maharashtra, Andhra Pradesh, Odisha, Jharkhand and Uttar Pradesh. Located in central India, the state comprises of 27 districts. Chhattisgarh is endowed with rich cultural heritage and is a home of some of India’s ancient caves, waterfalls, The river side view temples, Buddhist sites, rock paintings, Chhattisgarh contains the wildlife and hill plateaus. source of one of the most important rivers of the South Historically Chhattisgarh shares Asian peninsula—the Mahanadi. its boundaries with many kingdoms. This river originates in a village The reason mentioned in the Imperial near Raipur. -

DISTRICT SURVEY REPORT BALOD (Chhattisgarh) AS PER NOTIFICATION NO
2016 DISTRICT SURVEY REPORT BALOD (Chhattisgarh) AS PER NOTIFICATION NO. S.O. 141(E) NEW DELHI, 15TH JANUARY, 2016 OF MINISTRY OF ENVIRONMENT, FOREST AND CLIMATE CHANGE DIRECTORATE OF GEOLOGY AND MINING MINERAL RESOURCES DEPARTMENT Govt. of Chhattisgarh DGM [Type the company name] 04/06/2016 1 Note: The information provided in this report has been prepared from the existing and latest data collected from the concerned authorities. Although utmost care has been taken during editing and printing, still there may be some minor corrections for which the Directorate of Geology & Mining, Regional office Raipur Sonakhan Bhawan, cannot be held responsible. The queries in this regard are most welcome by the concerned office. 1 2 LOCATION MAP 2 3 3 4 4 5 1- INTRODUCTION The Balod district is one of the densely populated and situated in the central part of the Chhattisgarh State covers an area of 3527 sq km. It falls in Survey of India Degree Sheet Nos. 64C, 64D, 64G and 64H bounded by latitude 20°23' to 21°03' N and longitude 80°48' to 81°30 E. It is surrounded by Durg districts in the north, Rajnandgaon District in the west, Dhamtari districts in the east and Kanker district in the south. Balod is the district headquarters and is well connected by road and railway. National Highway No. 43 and connects Balod with Dhamtari. Balod is also connected by road with Dalli Rajhara, Patan, Dhamtari, Rajnandgaon and Durg with the other important towns in the district. Acording to Notification of Environment, Forest and Climate Change Ministry, Goverment of India dated15 January 2016, as prescribed in Para 7(iii) and Annexure (x).District Survey Report has been prepared. -
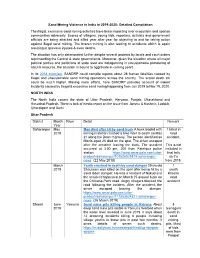
Sand Mining Violence in India in 2019-2020
Sand Mining Violence in India in 2019-2020: Detailed Compilation The illegal, excessive sand mining activities have been impacting river ecosystem and riparian communities adversely. Scores of villagers, young kids, reporters, activists and government officials are being attacked and killed year after year for objecting to and for taking action against illegal sand mining. The brazen mining is also leading to accidents which is again resulting in grievous injuries & even deaths. The situation has only deteriorated further despite several protests by locals and court orders reprimanding the Central & state governments. Moreover, given the situation where all major political parties and politicians at state level are indulgencing in unsustainable plundering of natural resource, the situation is bound to aggravate in coming years. In its 2018 overview, SANDRP could compile reports about 28 human fatalities caused by illegal and unsustainable sand mining operations across the country. The actual death toll could be much higher. Making more efforts, here SANDRP provides account of violent incidents caused by illegal & excessive sand mining happening from Jan 2019 to Nov 15, 2020. NORTH INDIA The North India covers the state of Uttar Pradesh, Haryana, Punjab, Uttarakhand and Himachal Pradesh. There is lack of media report on the issue from Jammu & Kashmir, Ladakh, Chandigarh and Delhi. Uttar Pradesh District Month River Detail Remark Year Saharanpur May Man died after hit by sand truck A truck loaded with 1 killed in 2018 mining material crushed a bike rider to death on May road 21 along the Doon Highway. The person identified as accident. Monis aged 25 died on the spot. -

Geomorphic Conditions, Archaeological - Architectural Heritage Structures and Conservation of Ancient Hindu Malhar Mud Fort Chattisgarh, India
ISSN: 2455-2631 © July 2021 IJSDR | Volume 6 Issue 7 GEOLOGIC- GEOMORPHIC CONDITIONS, ARCHAEOLOGICAL - ARCHITECTURAL HERITAGE STRUCTURES AND CONSERVATION OF ANCIENT HINDU MALHAR MUD FORT CHATTISGARH, INDIA Dr. HD.DIWAN*, Dr. S.S. BHADAURIA**, Dr. PRAVEEN KADWE***, Dr. D.SANYAL**** *Alumni, Department of Applied Geology, Deptt. Of Civil Engineering, NIT, Raipur, C.G., India **Head, Department of Geology, Govt. NPG College of Science Raipur, C.G. ***Head, Department of Defense Studies, Govt. NPG College of Science Raipur, C.G. ****Head, Department of Architecture, NIT, Raipur, C.G., India Abstract: The ancient trade route from Kausambi to the South - Eastern Sea Side i.e. Jagganath Puri, Odisha were followed via Bharhut, Bandhavgarh, Amarkantak of M.P. and Kharod, Malhar, Sirpur in C.G. The Malhar is located in Bilaspur district, CG towards South East, about 32 km. distance and lies in geographical Latitude North 21°55' and East longitude 82°20’ in Masturi Tahsil. Under Natural Heritages, the environment of Malhar is surrounded by three rivers, Arpa in the West, Lilagar in the East Shivnath in the South. The landscape shows undulating plains (250 m AMSL) with gentle slope towards lowlying, river drainage channels, due to presence of Sedimentary Horizontal Bedded (SHB) rock Formations of Chhattisgarh (Protereozoic) Supergroup. The excavations at Malhar by Archaealogical Survey of India (ASI) designated as ASI code - NCT - 17, and Archaeology Deptt. of C.G. State, considered continuous inhabitation, ancient civilization from Ancient period to Medieval period in Indian History. The ancient fort - Ramparts in Kosala Village and Ferry Potnar, still exists at present, indicates its antiquity to Pre-Mauryan reign period (600 BC). -

DISTRICT SURVEY REPORT of Balodabazar - Bhatapara
2016 DISTRICT SURVEY REPORT OF BalodaBazar - Bhatapara DIRECTORATE OF GEOLOGY AND MINING MINERAL RESOURCES DEPARTMENT Govt. of Chhattisgarh DGM 24/06/2016 Note: The information provided in this report has been prepared from the existing and latest data collected from the concerned authorities. Although utmost care has been taken during editing and printing, still there may be some minor corrections for which the Directorate of Geology & Mining, Regional office Raipur Sonakhan Bhawan, cannot be held responsible. The queries in this regard are most welcome by the concerned office. 1 LOCATION AND COMMUNICATION MAP – BALODA BAZAR DISTRICT CHHATTISGARH .•UTTAAf N DISTRICT MAP ,;_:,.,_,.., ,__ ,. _ __~'. .:- ~::'.': ?'·'--..~ JHARKHANO A ,,• . ,111,.,r,J -"' ... ....:i m..>1> t .., .... ._ \_ l((WIH'A WkAJPIM ( , ~tk~ hp" r:•;1:n.~,:, . ' ~ LMM~~~-......f ""'"° MADH'l'A PRADESM r-- ···•. \. .,· ....r,. ,.:-"' •,..., ( } , : k<l _ ot,. : ,~,. ' , .. ,;~~J~h!jf,;m1.;,,d . ·~; ® \ -~\ <,,! Bifd.00 V ' ~ \>lln.lb.\f!: OfUSSA LEGEND }-.:,;~::?~ -~\":~:+ ··· , •• . l)i;i JJl,3 Rotlll(~IV t;t.:tte Bound;,uy ·''\. J ~ll'll7'Up11t(• ? k0NUAG~)9:' ',,/ ··~.~) (! ) State Caoltsl .. ·~..- . : ~;,i ~YANPUR ~ ~ ti;\/),~ (i) Ois:riciHQ ,,.-·'\ .,_,:·:. ...t" ..... , ..J ) S- Basia, ;_'~/· ,.r MIAP VR ' • "\ tlll) TJIIII..,. ~ ~_,.,.::~./ ' °"'t.tt~·~ - -~;pu1.·: !' ~. ._ .;~~~-~s.;;~,, c,,,,.,.,,..": •,) ', _;.\ .. .. , ;t ANOHRA '. I U,;,,11! ~ PRAO<SH TUANGANA :~;: .;_ ~~ ( ' / ..., ....... Map not to Sea~ __l.., :·... .. J.~:.~ .:··· Copyright© ::>016 www.mapsoflndl -

Acta Biomedica Scientia
14 Manish Upadhyay and Anisha Mishra. / Acta Biomedica Scientia. 2014;1(1):14-17. e - ISSN – XXXX-XXXX Print ISSN - XXXX-XXXX Acta Biomedica Scientia Journal homepage: www. http://mcmed.us/journal/abs STUDY OF PHYSICO-CHEMICAL PROPERTIES OF SURFACE WATER (SHIVNATH RIVER & PONDS) IN DURG AND RAJNANDGAON REGION 1* 2 Manish Upadhyay and Anisha Mishra 1Head, Department of Chemistry, C.V.Raman University, Kota, Bilaspur, Chattisgarh, India. 2Research scholar, Department of Chemistry, C.V.Raman University, Kota, Bilaspur, Chattisgarh, India. Article Info ABSTRACT Received 25/08/2013 An investigation was undertaken to determine the Quality of surface water of shivnath river Revised 15/09/2013 & ponds in durg and rajnandgaon region”. The study assessed the evolution of water Accepted 18/10/2013 quality in Surface of Durg and Rajnandgaon district. A comparative study of both type of surface water i.e. river as well as pond water was carried out by taking certain important Key word: Surface parameters like temperature, pH, Total dissolved solids, alkalinity, dissolved oxygen, water, Investigation, chloride and the same were compared with drinking water standard IS:10500,2005. In this Community pond, present investigation it was found that the maximum parameters were not at the level of Parameters. pollution. So both type of surface water satisfy the requirement for the use in various purposes. But the study of pond water indicated that the community ponds are highly polluted and unsafe for human use. Temple pond is comparatively less polluted than small community pond and large community pond. INTRODUCTION (evapotranspiration), condensation, precipitation, and Water covers 70.9% of the Earth's surface, and is runoff, usually reaching the sea [1, 2, 3]. -

Page 1 © 2020 (JCRT | Volume 8, Issue 11 November 2020
www.ijcrt.org © 2020 IJCRT | Volume 8, Issue 11 November 2020 | ISSN: 2320-2882 Estimation of Water Quality Parameters of Shivnath River in Rajnandgoan District, Chhattisgarh, India Shraddha Vaishnav¹ Dr. Vishwaprakash Roy², Dr. Ashish Saraf ³ 1,2,3 MATS School of Sciences, MATS University, Raipur, C.G., India Abstract: Water quality parameters are key factor for the survival of any life forms on earth. The population of the country depends on water resources as it is the physical base of life. The quality of Shivnath River has become polluted due to the anthropogenic activities, Industrial effluents, West disposal etc. so An investigation was undertaken to determine the quality of Shivnath River water by analyzing various Physico-chemical parameter like Temperature, Odour, Color, TDS, DO,COD, BOD, and biological parameters like faecal coliforms. It was found that the maximum parameters were indicated that the river water is polluted and unsafe for human use. Key words –TDS, BOD, COD, Coliforms. 1 Introduction Water is important gift of nature to all living things on Earth. We cannot imagine A life without water. On Earth surface water covers 70.9% and 96.5% of the planet water found in Ocean, in glacier 1.7% in ground water 1.7%and 0.001% in the air as vapor and % of precipitation is only 2.5% on Earth 98.8% of the Water is in ice and groundwater and only 0.3% of all freshwater is in rivers, lakes and atmosphere. Hydrological cycle facilitate the movement of water on Earth that make it available for all livings in different- different forms (10).