Identification and Biosynthesis of Acylphloroglucinols in Hypericum Gentianoides Matthew .C Crispin Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chemistry, Pharmacoligy and Clinical Properties of Heracleum Persicuam
African Journal of Pharmacy and Pharmacology Vol. 6(19), pp. 1387-1394, 22 May, 2012 Available online at http://www.academicjournals.org/AJPP DOI: 10.5897/AJPP12.248 ISSN 1996-0816 ©2012 Academic Journals Review Phytochemistry, pharmacology and medicinal properties of Hypericum perforatum L. Jinous Asgarpanah Department of Pharmacognosy, Pharmaceutical Sciences Branch, Islamic Azad University (IAU), Tehran, Iran. E-mail: [email protected]. Tel: 22640051. Fax: 22602059. Accepted 23 April, 2012 Hypericum perforatum is known as St. John's Wort. H. perforatum extracts and essential oil are important in drug development with numerous pharmacological activities around the world, including Iran. For a long time, H. perforatum has been used in traditional medicines for healing skin wounds, eczema, burns, diseases of the alimentary tract, and psychological disorders especially depression. H. perforatum has recently been shown to have antioxidant, anticonvulsant, analgesic, anti-inflammatory, cytotoxic and antidiabetic activities. Hypericin, pseudohypericin, hyperoside, rutin, quercetin and hyperforin are the main compounds which are reported in this plant. α-Pinene, caryophyllene, caryophyllene oxide, germacrene D and 2-methyloctane were identified as the major constituents for H. perforatum essential oil collected from different parts of the world. Due to the easy collection of the plant, its widespread and also remarkable biological activities, this plant has become a medicine worldwide. This review presents comprehensive analyzed information on the botanical, chemical and pharmacological aspects of H. perforatum at preclinical and clinical levels. Key words: Hypericum perforatum, hypericaceae, hypericin, antidepressant. INTRODUCTION Hypericum perforatum, commonly known as St. John's branches, linear-oblong, non-toothed, covered with Wort is a flowering plant and is a native from Europe and translucent glands (Figure 2). -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Explosive Radiation in High Andean Hypericum—Rates of Diversification
ORIGINAL RESEARCH ARTICLE published: 11 September 2013 doi: 10.3389/fgene.2013.00175 Explosive radiation in high Andean Hypericum—rates of diversification among New World lineages Nicolai M. Nürk 1*, Charlotte Scheriau 1 and Santiago Madriñán 2 1 Department of Biodiversity and Plant Systematics, Centre for Organismal Studies Heidelberg, Heidelberg University, Heidelberg, Germany 2 Laboratorio de Botánica y Sistemática, Departamento de Ciencias Biológicas, Universidad de los Andes, Bogotá DC, Colombia Edited by: The páramos, high-elevation Andean grasslands ranging from ca. 2800 m to the snow Federico Luebert, Freie Universität line, harbor one of the fastest evolving biomes worldwide since their appearance in the Berlin, Germany northern Andes 3–5 million years (Ma) ago. Hypericum (St. John’s wort), with over 65% Reviewed by: of its Neotropical species, has a center of diversity in these high Mountain ecosystems. Andrea S. Meseguer, Institute National de la research agricultural, Using nuclear rDNA internal transcribed spacer (ITS) sequences of a broad sample of France New World Hypericum species we investigate phylogenetic patterns, estimate divergence Colin Hughes, University of Zurich, times, and provide the first insights into diversification rates within the genus in the Switzerland Neotropics. Two lineages appear to have independently dispersed into South America *Correspondence: around 3.5 Ma ago, one of which has radiated in the páramos (Brathys). We find strong Nicolai M. Nürk, Department of Biodiversity and Plant Systematics, support for the polyphyly of section Trigynobrathys, several species of which group within Centre for Organismal Studies Brathys, while others are found in temperate lowland South America (Trigynobrathys Heidelberg, Heidelberg University, s.str.). -

Winter 2014-2015 (22:3) (PDF)
Contents NATIVE NOTES Page Fern workshop 1-2 Wavey-leaf basket Grass 3 Names Cacalia 4 Trip Report Sandstone Falls 5 Kate’s Mountain Clover* Trip Report Brush Creek Falls 6 Thank yous memorial 7 WEST VIRGINIA NATIVE PLANT SOCIETY NEWSLETTER News of WVNPS 8 VOLUME 22:3 WINTER 2014-15 Events, Dues Form 9 Judy Dumke-Editor: [email protected] Phone 740-894-6859 Magnoliales 10 e e e visit us at www.wvnps.org e e e . Fern Workshop University of Charleston Charleston WV January 17 2015, bad weather date January 24 2015 If you have thought about ferns, looked at them, puzzled over them or just want to know more about them join the WVNPS in Charleston for a workshop led by Mark Watson of the University of Charleston. The session will start at 10 A.M. with a scheduled end point by 12:30 P.M. A board meeting will follow. The sessions will be held in the Clay Tower Building (CTB) room 513, which is the botany lab. If you have any pressed specimens to share, or to ask about, be sure to bring them with as much information as you have on the location and habitat. Even photographs of ferns might be of interest for the session. If you have a hand lens that you favor bring it along as well. DIRECTIONS From the North: Travel I-77 South or 1-79 South into Charleston. Follow the signs to I-64 West. Take Oakwood Road Exit 58A and follow the signs to Route 61 South (MacCorkle Ave.). -

Moorhead Ph 1 Final Report
Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. 4. Title and Subtitle 5. Report Date Ecological Assessment of a Wetlands Mitigation Bank August 2001 (Phase I: Baseline Ecological Conditions and Initial Restoration Efforts) 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Kevin K. Moorhead, Irene M. Rossell, C. Reed Rossell, Jr., and James W. Petranka 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Departments of Environmental Studies and Biology University of North Carolina at Asheville Asheville, NC 28804 11. Contract or Grant No. 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered U.S. Department of Transportation Final Report Research and Special Programs Administration May 1, 1994 – September 30, 2001 400 7th Street, SW Washington, DC 20590-0001 14. Sponsoring Agency Code 15. Supplementary Notes Supported by a grant from the U.S. Department of Transportation and the North Carolina Department of Transportation, through the Center for Transportation and the Environment, NC State University. 16. Abstract The Tulula Wetlands Mitigation Bank, the first wetlands mitigation bank in the Blue Ridge Province of North Carolina, was created to compensate for losses resulting from highway projects in western North Carolina. The overall objective for the Tulula Wetlands Mitigation Bank has been to restore the functional and structural characteristics of the wetlands. Specific ecological restoration objectives of this Phase I study included: 1) reestablishing site hydrology by realigning the stream channel and filling drainage ditches; 2) recontouring the floodplain by removing spoil that resulted from creation of the golf ponds and dredging of the creek; 3) improving breeding habitat for amphibians by constructing vernal ponds; and 4) reestablishing floodplain and fen plant communities. -

Assessment Report on Hypericum Perforatum L., Herba
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101303/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) ASSESSMENT REPORT ON HYPERICUM PERFORATUM L., HERBA 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 51 E-mail: [email protected] http://www.emea.europa.eu © European Medicines Agency, 2009. Reproduction is authorised provided the source is acknowledged TABLE OF CONTENTS I. REGULATORY STATUS OVERVIEW...................................................................................4 II. ASSESSMENT REPORT............................................................................................................5 II.1 INTRODUCTION..........................................................................................................................6 II.1.1 Description of the herbal substance(s), herbal preparation(s) or combinations thereof 6 II.1.1.1 Herbal substance:........................................................................................................ 6 II.1.1.2 Herbal preparation(s): ................................................................................................ 7 II.1.1.3 Combinations of herbal substance(s) and/or herbal preparation(s)........................... 9 Not applicable. ................................................................................................................................9 II.1.1.4 Vitamin(s) ................................................................................................................... -

Toxicity Assessment of Hypericum Olympicum Subsp. Olympicum L. On
J Appl Biomed journal homepage: http://jab.zsf.jcu.cz DOI: 10.32725/jab.2020.002 Journal of Applied Biomedicine Original research article Toxicity assessment of Hypericum olympicum subsp. olympicum L. on human lymphocytes and breast cancer cell lines Necmiye Balikci 1, Mehmet Sarimahmut 1, Ferda Ari 1, Nazlihan Aztopal 1, 2, Mustafa Zafer Özel 3, Engin Ulukaya 1, 4, Serap Celikler 1 * 1 Uludag University, Faculty of Science and Arts, Department of Biology, Bursa, Turkey 2 Istinye University, Faculty of Science and Literature, Department of Molecular Biology and Genetics, Istanbul, Turkey 3 University of York, Department of Chemistry, Heslington, York, United Kingdom 4 Istinye University, Faculty of Medicine, Department of Medical Biochemistry, Istanbul, Turkey Abstract There is a limited number of studies about the constituents ofHypericum olympicum subsp. olympicum and its genotoxic and cytotoxic potency. We examined the possible antigenotoxic/genotoxic properties of methanolic extract of H. olympicum subsp. olympicum (HOE) on human lymphocytes by employing sister chromatid exchange, micronucleus and comet assay and analyzed its chemical composition by GCxGC-TOF/MS. The anti-growth activity against MCF-7 and MDA-MB-231 cell lines was assessed by using the ATP viability assay. Cell death mode was investigated with fluorescence staining and ELISA assays. The major components of the flower and trunk were determined as eicosane, heptacosane, 2-propen-1-ol, hexahydrofarnesyl acetone and α-muurolene. HOE caused significant DNA damage at selected doses (250–750 µg/ml) while chromosomal damage was observed at higher concentrations (500 and 750 µg/ml). HOE demonstrated anti-growth activity in a dose-dependent manner between 3.13–100 µg/ml. -

Response of Two Chrysolina Species to Different Hypericum Hosts
Seventeenth Australasian Weeds Conference Response of two Chrysolina species to different Hypericum hosts Ronny Groenteman', Simon V. Fowler' and Jon J. Sullivan2 ' Landcare Research, Gerald Street, PO Box 40, Lincoln, New Zealand 2Bio-Protection Research Centre, PO Box 84, Lincoln University, Lincoln, New Zealand Corresponding author: [email protected] SummaryChrysolina hyperici and C. quadrigemina introduced in 1963 but, for many years was thought (Coleoptera: Chrysomelidae) were introduced to to have failed to establish (reviewed by Hancox et al. New Zealand for biological control of St John's wort 1986). Chrysolina quadrigemina was rediscovered in (SJW), Hypericum perforatum, following successful the late 1980s (Fraser and Emberson 1987). It is now biological control in Australia. In other parts of the abundant in mixed populations with C. hyperici (R. invaded range of SJW worldwide C. quadrigemina is Groenteman personal observations). generally accepted as the more significant contributor St John's wort beetles are not strictly restricted to to SJW successful biocontrol. Their ability to feed and H. perforatum, and are known to be able to develop on develop on indigenous Hypericum species was not other Hypericum species (some examples are reviewed tested. Chrysolina hyperici established well while C. by Harris 1988). New Zealand hosts 10 naturalised quadrigemina was initially thought to have failed to es- (Healy 1972) and four indigenous (Heenan 2008) tablish in New Zealand, although it is now widespread. Hypericum species. Little is known about the suit- Thus, identifying differences between Chrysolina spe- ability and impacts of either Chrysolina species on cies in host preference and performance would have these Hypericum species. -

Millepertuis (Hypericum) – Famille : Hypericacées
Millepertuis (Hypericum) – Famille : Hypericacées Les millepertuis utilisés pour l’ornement de nos jardins sont uniquement constitués de petits arbustes dont la hauteur s’échelonne d’une cinquantaine de centimètres à environ 1,20 m. Cependant, il en existe quelques uns qui se présentent comme de véritables arbustes ou petits arbres, mais également de nombreuses plantes vivaces ou annuelles. Leur intérêt est essentiellement porté par leurs fleurs jaunes possédant majoritairement 5 pétales, à leurs très nombreuses étamines, parfois à leur fruits constitués de capsules. Principe de ramification La très grande majorité des millepertuis se régénèrent naturellement par la formation de nouvelles pousses issues directement de la souche, de la base des rameaux (basitonie), ou parfois issues de la partie médiane (arcures). Exceptionnellement, les nouvelles pousses ne se forment qu’en extrémité des rameaux préexistants (acrotonie). Principe de floraison Tous les millepertuis laissent apparaître leur floraison en extrémité des pousses formées au cours de l’année. Parfois, la floraison est cependant plus abondante sur les bois courts issus de ramifications secondaires formées sur des bois plus âgés. La taille des millepertuis Hypericum inodorum ‘Elstead’ Bien que ce millepertuis soit capable de former une mini charpente, la floraison et la qualité du feuillage ne sont jamais si belles que quand les plantes sont rabattues totalement annuellement, en fin d’hiver. Taille de plantation Rabattre les plantes à quelques centimètres du sol. En l’absence de taille (photo de gauche), Hypericum inodorum ‘Elstead’ se dégarnit très vite et son port présente peu d’intérêt. Rabattu sur la souche chaque année, il développe des rameaux vigoureux, un feuillage sain, et présente de délicates inflorescences jaunes. -
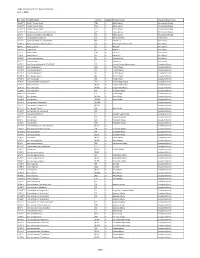
UDBG Inventory of Tree and and Shrubs June 1, 2018 Page 1
UDBG Inventory of Tree and and Shrubs June 1, 2018 Acc. Num Scientific Name Location Mapped Common Name Family Common Name 15-167*3 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*2 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*1 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 01-144*1 Abelia x grandiflora [Confetti] = 'Conti' GH Y Glossy Abelia Honeysuckle Family 02-2*1 Abelia x grandiflora 'Little Richard' F5 N Glossy Abelia Honeysuckle Family 70-1*1 Abeliophyllum distichum C3 N White Forsythia Olive Family 15-57*1 Abies balsamea var. phanerolepis NBF Y Balsam Fir Pine Family 90-47*1 Abies cephalonica 'Meyer's Dwarf' C2 N Meyer's Dwarf Grecian Fir Pine Family 89-4*1 Abies concolor C1 N White Fir Pine Family 01-74*1 Abies firma C1 N Momi Fir Pine Family 12-9*7 Abies fraseri PILL N Fraser Fir Pine Family 71-1*2 Abies koreana C1 N Korean Fir Pine Family 95-26*1 Abies nordmanniana C1 N Nordmann Fir Pine Family 96-21*1 Abies pinsapo C1 N Spanish Fir Pine Family 14-72*1 Acer [Crimson Sunset] = 'JFS-KW202' FE Y Crimson Sunset hybrid maple Soapberry Family 88-40*1 Acer buergerianum W2 N Trident Maple Soapberry Family 91-32*1 Acer buergerianum C3 N Trident Maple Soapberry Family 92-78*1 Acer buergerianum A2 Y Trident Maple Soapberry Family 92-78*2 Acer buergerianum A2 Y Trident Maple Soapberry Family 97-43*1 Acer campestre C3 N Hedge Maple Soapberry Family 94-95*1 Acer campsetre 'Compactum' WV1 N Dwarf Hedge Maple Soapberry Family 93-41*1 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 93-41*2 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 91-58*1 Acer cissifolium F2 Y Ivy-leafed Maple Soapberry Family 98-123*1 Acer davidii FH Y David Maple Soapberry Family 94-13*1 Acer ginnala NUR30 N Amur Maple Soapberry Family 94-13*2 Acer ginnala NUR30 N Amur Maple Soapberry Family 93-56*1 Acer ginnala 'Compactum' NUR29 N Soapberry Family 93-56*2 Acer ginnala 'Compactum' NUR29 N Soapberry Family 97-11*1 Acer ginnala 'Flame' C3 N Amur Maple Soapberry Family 97-13*1 Acer ginnala var. -

The Impact of Climate Change on Hypericum Elodes L
Atti Soc. Tosc. Sci. Nat., Mem., Serie B, 121 (2014) pagg. 15-24, figg. 3; doi: 10.2424/ASTSN.M.2014.02 ANGELINO CARTA (*) THE IMPACT OF CLIMATE CHANGE ON HYPERICUM ELODES L. (HYPERICACEAE) DISTRIBUTION: PREDICTING FUTURE TRENDS AND IDENTIFYING PRIORITIES Abstract - The Impact of Climate Change on Hypericum elodes L. termine (2050), che rappresentano le priorità di conservazione ex situ; (Hypericaceae) distribution: Predicting Future Trends and Identifying (2) identifica località centrali che potrebbero resistere al cambiamento Priorities. In this study the present and future predicted distribu- climatico almeno fino al 2050, e quindi servire come riservein situ a tion of the Atlantic-European soft-water pools specialist Hypericum lungo termine per H. elodes. elodes L. (Hypericaceae) is modelled in order to facilitate appro- priate decision making for conservation, monitoring and future Parole chiave - Cambiamenti climatici, Hypericum, MaxEnt, Modelli research. Using the methods of Maximum Entropy the future dis- distributivi. tribution has been examined with the HadCM3 climate model over the year 2050. H. elodes is confirmed as a climate-sensitive species, with a W-Eu- ropean distribution and preferences for acid substrates. The model INTRODUCTION shows a marked negative influence of climate change onH. elodes. In a locality analysis the outcome is a c. 58% reduction in the number The observation of ecological properties of species of pre-existing bioclimatically suitable localities by 2050. In an area and their areas of distribution being related is not analysis the outcome is a 57% reduction in suitable bioclimatic space new (Humboldt & Bonpland, 1807; Watson, 1835), by 2050. but the increasing availability of information on the This study establishes a fundamental baseline for assessing the con- sequences of climate change on populations of H. -

Vegetation Classification and Mapping Project Report
USGS – NPS Vegetation Mapping Program Allegheny Portage Railroad National Historic Site National Park Service U.S. Department of the Interior Northeast Region Philadelphia, Pennsylvania Vegetation Classification and Mapping at Allegheny Portage Railroad National Historic Site Technical Report NPS/NER/NRTR—2007/079 USGS – NPS Vegetation Mapping Program Allegheny Portage Railroad National Historic Site ON THE COVER Allegheny Hardwood Forest in Allegheny Portage Railroad National Historic Site. Photograph by: Ephraim Zimmerman. USGS – NPS Vegetation Mapping Program Allegheny Portage Railroad National Historic Site Vegetation Classification and Mapping at Allegheny Portage Railroad National Historic Site Technical Report NPS/NER/NRTR--2006/079 Stephanie J. Perles1, Gregory S. Podniesinski1, Ephraim A. Zimmerman1, Elizabeth Eastman 2, and Lesley A. Sneddon3 1 Pennsylvania Natural Heritage Program Western Pennsylvania Conservancy 208 Airport Drive Middletown, PA 17057 2 Center for Earth Observation North Carolina State University 5112 Jordan Hall, Box 7106 Raleigh, NC 27695 3 NatureServe 11 Avenue de Lafayette, 5th Floor Boston, MA 02111 March 2007 U.S. Department of the Interior National Park Service Northeast Region Philadelphia, Pennsylvania i USGS – NPS Vegetation Mapping Program Allegheny Portage Railroad National Historic Site The Northeast Region of the National Park Service (NPS) comprises national parks and related areas in 13 New England and Mid-Atlantic states. The diversity of parks and their resources are reflected in their designations as national parks, seashores, historic sites, recreation areas, military parks, memorials, and rivers and trails. Biological, physical, and social science research results, natural resource inventory and monitoring data, scientific literature reviews, bibliographies, and proceedings of technical workshops and conferences related to these park units are disseminated through the NPS/NER Technical Report (NRTR) and Natural Resources Report (NRR) series.