ICD-10-PCS Procedure Coding Trends
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Surgical Treatment of Snoring and Obstructive Sleep Apnea Syndrome
Medical Policy Surgical Treatment of Snoring and Obstructive Sleep Apnea Syndrome Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information Policy History Policy Number: 130 BCBSA Reference Number: 7.01.101 Related Policies None Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Medicare HMO BlueSM and Medicare PPO BlueSM Members Uvulopalatopharyngoplasty (UPPP) may be MEDICALLY NECESSARY for the treatment of clinically significant obstructive sleep apnea syndrome (OSAS) in appropriately selected adult patients who have failed an adequate trial of continuous positive airway pressure (CPAP) or failed an adequate trial of an oral appliance (OA). Clinically significant OSA is defined as those patients who have: Apnea/hypopnea Index (AHI) or Respiratory Disturbance Index (RDI) 15 or more events per hour, or AHI or RDI 5 or more events and 14 or less events per hour with documented symptoms of excessive daytime sleepiness, impaired cognition, mood disorders or insomnia, or documented hypertension, ischemic heart disease, or history of stroke. Hyoid suspension, surgical modification of the tongue, and/or maxillofacial surgery, including mandibular- maxillary advancement (MMA), may be MEDICALLY NECESSARY in appropriately selected adult patients with clinically significant OSA and objective documentation of hypopharyngeal obstruction who have failed an adequate trial of continuous positive airway pressure (CPAP) or failed an adequate trial of an oral appliance (OA). Clinically significant OSA is defined as those patients who have: AHI or RDI 15 or more events per hour, or AHI or RDI 5 or more events and 14 or less events per hour with documented symptoms of excessive daytime sleepiness, impaired cognition, mood disorders or insomnia, or documented hypertension, ischemic heart disease, or history of stroke. -

Urology 1 Cystoscopes
Urology 1 Cystoscopes 2 Urethrotomes 3 Resectoscopes 4 Uretero-Renoscopes 5 Nephroscopes 6 Lithotripsy (UreTron) 7 Laser Therapy 8 Small Caliber 9 Fluid Management 10 Accessories Richard Wolf Medical Instruments Corporation assumes no responsibility or liability for any errors or omissions in the content of this catalog. The information contained in this catalog is provided on an “as is” basis with no guarantees of completeness, accuracy, usefulness, or timeliness, and without warranties of any kind whatsoever, expressed or implied. 1777- 02.01-1118USA Cysto-Urethroscopes 8650 E-line design CYSTOSCOPES The E-line design guarantees optimum handling and safe, fatigue-free operation as well as a wide range of possible combinations. Basic Set for Cystoscopy Cysto-urethroscope sheath, 19.5 Fr. with obturator 8650.0341 Adapter with 1 instrument port 8650.264 Insert with Albarran deflector, 2 instrument ports 8650.204 Sterile universal sealing valve (pack of 5) 4712348 Viewing obturator 8650.724 Biopsy forceps Marburg 8650.614 Grasping forceps 8650.684 PANOVIEW telescope, 0° 8650.414 PANOVIEW telescope, 70° 8650.415 Otis urethrotome 8517.00 822.31 822.13 822.31 Flexible connector 822.13 Tray 8585030 1777- 02.01-1118USA 2 PANOVIEW Telescopes Overview CYSTOSCOPES Ø Viewing direction Color code Application mm 0° blue Standard 4 8650.414 12° orange Standard 4 8654.431 Standard 4 8654.422 30° red Long sheath 4 8668.433* 70° yellow Standard 4 8650.415 * Only 25° available. 1777- 02.01-1118USA 3 Cysto-Urethroscope 8650 for telescope 4 mm, 0°, 12°, 30°, 70° and adapters CYSTOSCOPES Sheaths and obturators Adapters Sheath incl. -
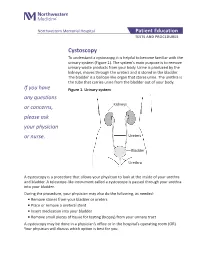
Cystoscopy to Understand a Cystoscopy, It Is Helpful to Become Familiar with the Urinary System (Figure 1)
Northwestern Memorial Hospital Patient Education TESTS AND PROCEDURES Cystoscopy To understand a cystoscopy, it is helpful to become familiar with the urinary system (Figure 1). The system’s main purpose is to remove urinary waste products from your body. Urine is produced by the kidneys, moves through the ureters and is stored in the bladder. The bladder is a balloon-like organ that stores urine. The urethra is the tube that carries urine from the bladder out of your body. If you have Figure 1. Urinary system any questions or concerns, Kidneys please ask your physician or nurse. Ureters Bladder Urethra A cystoscopy is a procedure that allows your physician to look at the inside of your urethra and bladder. A telescope-like instrument called a cystoscope is passed through your urethra into your bladder. During the procedure, your physician may also do the following, as needed: ■ Remove stones from your bladder or ureters ■ Place or remove a ureteral stent ■ Insert medication into your bladder ■ Remove small pieces of tissue for testing (biopsy) from your urinary tract A cystoscopy may be done in a physician’s office or in the hospital’s operating room (OR). Your physician will discuss which option is best for you. Preparation and procedure If the test is done in the OR, you will be asked to sign a written consent. The OR procedure and any special preparation will be explained to you. There may be some discomfort during the examination. Some patients may require sedation or anesthesia. Depending on the type of medication used for your procedure, you will be told if you need to stop eating and drinking before your procedure. -

Bris Or Brit Milah (Ritual Circumcision) According to Jewish Law, a Healthy Baby Boy Is Circumcised on the Eighth Day After His Birth
Bris or Brit milah (ritual circumcision) According to Jewish law, a healthy baby boy is circumcised on the eighth day after his birth. The brit milah, the ritual ceremony of removing the foreskin which covers the glans of the penis, is a simple surgical procedure that can take place in the home or synagogue and marks the identification of a baby boy as a Jew. The ceremony is traditionally conducted by a mohel, a highly trained and skilled individual, although a rabbi in conjunction with a physician may perform the brit milah. The brit milah is a joyous occasion for the parents, relatives and friends who celebrate in this momentous event. At the brit milah, it is customary to appoint a kvater (a man) and a kvaterin (a woman), the equivalent of Jewish godparents, whose ritual role is to bring the child into the room for the circumcision. Another honor bestowed on a family member is the sandak, who is most often the baby’s paternal grandfather or great-grandfather. This individual traditionally holds the baby during the circumcision ceremony. The service involves a kiddush (prayer over wine), the circumcision, blessings, a dvar torah (a small teaching of the Torah) and the presentation of the Jewish name selected for the baby. During the brit milah, a chair is set aside for Elijah the prophet. Following the ceremony, a seudat mitzvah (celebratory meal) is available for the guests. Please take note: Formal invitations for a bris are not sent out. Typically, guests are notified by phone or email. The baby’s name is not given before the bris. -

Cardiac Checklist Connecticare
Cardiac Checklist ConnectiCare Please be prepared to provide the applicable information from the following list when requesting prior authorization for a cardiac procedure managed by Magellan Healthcare1: 1. Medical chart notes – all notes from patient chart related to the requested procedure, including patient’s current cardiac status/symptoms, cardiac factors and indications. 2. Relevant patient information, including: a. Patient age, height, weight, and BMI. b. Family history of heart problems (including relationship to member, age at diagnosis, type of event, etc.). c. Medical history (e.g. diabetes, hypertension, stroke, arrhythmia, etc.). d. Cardiac risk factors. e. Previous cardiac treatments, surgeries or interventions (medications, CABG, PTCA, stent, heart valve surgery, pacemaker/defibrillator insertion, surgery for congenital heart disease, etc.). f. Problems with exercise capacity (orthopedic, pulmonary, or peripheral vascular disease; distance, heart rate). 3. Diagnostic or imaging reports from previous tests (exercise stress test, echocardiography, stress echocardiography, MPI, coronary angiography, etc.). a. For pacemaker or Implantable Cardioverter Defibrillator (ICD) requests, include EKG and/or telemetry strips showing bradycardia, EKG showing conduction abnormalities, EP study report, and/or tilt table test report, if applicable. b. For cardiac resynchronization therapy requests, include left ventricular function test report indicating LVEF, documentation of CHF symptoms and NYHA class and/or 12-Lead EKG showing QRS width, if applicable. c. For cardiac catheterization requests, include EKG results showing relevant changes, left ventricular function test reports, documentation of recent ejection fraction, etc. d. Cardiac catheterization requests also require the submission of digital images (e.g. DICOM files) from previous procedures. The digital image from a previous MPI, Stress Echocardiography, Heart PET or other cardiac catheterization is considered to be relevant and necessary clinical information. -

EARLY DETECTION Breast Health Awareness and Clinical Breast Exam
EARLY DETECTION Breast Health Awareness and Clinical Breast Exam Knowledge Summary EARLY DETECTION Breast Health Awareness and Clinical Breast Exam INTRODUCTION KEY SUMMARY Early diagnosis of breast cancer begins with the establish- Early detection programs ment of programs to improve early detection of symptomatic ¬ Early diagnosis of breast cancer can improve survival, lower women, or women with breast lumps that patients and their morbidity and reduces the cost of care when followed by a providers can feel. Early recognition of symptoms and accu- prompt diagnosis and effective treatment. rate diagnosis of breast cancer can result in cancers being diagnosed at earlier stages when treatment is more feasible, ¬ An effective early diagnosis program includes: affordable and effective. This requires that health systems √ Breast health awareness education. have trained frontline personnel who are able to recognize the √ Reducing barriers to accessing care. signs and symptoms of breast abnormalities for both benign √ Clinical breast exam (CBE) performed by primary care breast issues as well as cancers, perform clinical breast exam providers. (CBE) and know the proper referral protocol when diagnostic √ Timely diagnosis for all women found to have abnormal workup is warranted. Women who can identify breast abnor- findings and timely treatment for all women proven by malities, who have timely access to health clinical evaluation, tissue diagnosis to have breast cancer. diagnosis and treatment and who are empowered to seek this √ If supported by evidence, a quality screening mammogra- care are more likely to be diagnosed at an earlier stage (see phy program performed in a cost-effective, resource-sus- Planning: Improving Access to Breast Cancer Care). -

Breast Scintimammography
CLINICAL MEDICAL POLICY Policy Name: Breast Scintimammography Policy Number: MP-105-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 11/23/2020 Issue Date: 11/23/2020 Effective Date: 12/21/2020 Next Annual Review: 10/2021 Revision Date: 09/16/2020 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 5 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ does not provide coverage in the Company’s Medicaid products for breast scintimammography. The service is considered experimental and investigational in all applications, including but not limited to use as an adjunct to mammography or in staging the axillary lymph nodes. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-105-MD-PA Page 1 of 5 DEFINITIONS Prior Authorization Review Panel – A panel of representatives from within the Pennsylvania Department of Human Services who have been assigned organizational responsibility for the review, approval and denial of all PH-MCO Prior Authorization policies and procedures. Scintimammography A noninvasive supplemental diagnostic testing technology that requires the use of radiopharmaceuticals in order to detect tissues within the breast that accumulate higher levels of radioactive tracer that emit gamma radiation. -

Correlation Between Echocardiography and Cardiac Catheterization for the Assessment of Pulmonary Hypertension in Pediatric Patients
Open Access Original Article DOI: 10.7759/cureus.5511 Correlation between Echocardiography and Cardiac Catheterization for the Assessment of Pulmonary Hypertension in Pediatric Patients Arshad Sohail 1 , Hussain B. Korejo 1 , Abdul Sattar Shaikh 2 , Aliya Ahsan 1 , Ram Chand 1 , Najma Patel 3 , Musa Karim 4 1. Pediatric Cardiology, National Institute of Cardiovascular Diseases, Karachi, PAK 2. Cardiology, National Institute of Cardiovascular Disease, Karachi, PAK 3. Paediatric Cardiology, National Institute of Cardiovascular Diseases, Karachi, PAK 4. Miscellaneous, National Institute of Cardiovascular Diseases, Karachi, PAK Corresponding author: Musa Karim, [email protected] Abstract Introduction Cardiac catheterization is widely considered the “gold standard” for the diagnosis of pulmonary hypertension. However, its routine use is limited due to its invasive nature. Therefore, the aim of this study was to evaluate the correlation between pulmonary artery pressures obtained by various parameters of transthoracic echocardiography and cardiac catheterization. Methods This study includes 50 consecutive patients with intracardiac shunt lesions diagnosed with severe pulmonary hypertension on echocardiography and admitted for cardiac catheterization at the National Institute of Cardiovascular Diseases (NICVD) in Karachi, Pakistan. Cardiac catheterization and transthoracic echocardiography were performed in all patients simultaneously and systolic (sPAP) and mean pulmonary artery pressure (mPAP) were assessed with both modalities. Correlations -

Breast Reconstruction with Expanders and Implants
Evidence-Based Clinical Practice Guideline: Breast Reconstruction with Expanders and Implants INTRODUCTION Disclaimer Evidence-based guidelines are strategies for patient management, The American Cancer Society estimates that nearly 230,000 American developed to assist physicians in clinical decision making. This women were diagnosed with invasive breast cancer in 2011.1 Many of guideline was developed through a comprehensive review of the these individuals will require mastectomy and total reconstruction of scientific literature and consideration of relevant clinical experience, the breast. The diagnosis and subsequent process can create signifi- and describes a range of generally acceptable approaches to diagnosis, cant confusion and distress for the affected persons and their families management, or prevention of specific diseases or conditions. This and, consequently, surgical treatment and reconstructive procedures guideline attempts to define principles of practice that should are of utmost importance in the breast cancer care continuum. In generally meet the needs of most patients in most circumstances. 2011, the American Society of Plastic Surgeons® (ASPS) reported an increase in the rate of breast reconstructions, citing nearly 100,000 However, this guideline should not be construed as a rule, nor procedures, of which the majority employed expanders/implants.2 should it be deemed inclusive of all proper methods of care The 3% increase in reconstructions over the course of just one year or exclusive of other methods of care reasonably directed at highlights the significance of maintaining patient safety and obtaining the appropriate results. It is anticipated that it will be optimizing surgical outcomes. necessary to approach some patients’ needs in different ways. -

Guidelines on Paediatric Urology S
Guidelines on Paediatric Urology S. Tekgül (Chair), H.S. Dogan, E. Erdem (Guidelines Associate), P. Hoebeke, R. Ko˘cvara, J.M. Nijman (Vice-chair), C. Radmayr, M.S. Silay (Guidelines Associate), R. Stein, S. Undre (Guidelines Associate) European Society for Paediatric Urology © European Association of Urology 2015 TABLE OF CONTENTS PAGE 1. INTRODUCTION 7 1.1 Aim 7 1.2 Publication history 7 2. METHODS 8 3. THE GUIDELINE 8 3A PHIMOSIS 8 3A.1 Epidemiology, aetiology and pathophysiology 8 3A.2 Classification systems 8 3A.3 Diagnostic evaluation 8 3A.4 Disease management 8 3A.5 Follow-up 9 3A.6 Conclusions and recommendations on phimosis 9 3B CRYPTORCHIDISM 9 3B.1 Epidemiology, aetiology and pathophysiology 9 3B.2 Classification systems 9 3B.3 Diagnostic evaluation 10 3B.4 Disease management 10 3B.4.1 Medical therapy 10 3B.4.2 Surgery 10 3B.5 Follow-up 11 3B.6 Recommendations for cryptorchidism 11 3C HYDROCELE 12 3C.1 Epidemiology, aetiology and pathophysiology 12 3C.2 Diagnostic evaluation 12 3C.3 Disease management 12 3C.4 Recommendations for the management of hydrocele 12 3D ACUTE SCROTUM IN CHILDREN 13 3D.1 Epidemiology, aetiology and pathophysiology 13 3D.2 Diagnostic evaluation 13 3D.3 Disease management 14 3D.3.1 Epididymitis 14 3D.3.2 Testicular torsion 14 3D.3.3 Surgical treatment 14 3D.4 Follow-up 14 3D.4.1 Fertility 14 3D.4.2 Subfertility 14 3D.4.3 Androgen levels 15 3D.4.4 Testicular cancer 15 3D.5 Recommendations for the treatment of acute scrotum in children 15 3E HYPOSPADIAS 15 3E.1 Epidemiology, aetiology and pathophysiology -

Synovial Fluidfluid 11
LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 253 Aptara Inc CHAPTER SynovialSynovial FluidFluid 11 Key Terms ANTINUCLEAR ANTIBODY ARTHROCENTESIS BULGE TEST CRYSTAL-INDUCED ARTHRITIS GROUND PEPPER HYALURONATE MUCIN OCHRONOTIC SHARDS RHEUMATOID ARTHRITIS (RA) RHEUMATOID FACTOR (RF) RICE BODIES ROPE’S TEST SEPTIC ARTHRITIS Learning Objectives SYNOVIAL SYSTEMIC LUPUS ERYTHEMATOSUS 1. Define synovial. VISCOSITY 2. Describe the formation and function of synovial fluid. 3. Explain the collection and handling of synovial fluid. 4. Describe the appearance of normal and abnormal synovial fluids. 5. Correlate the appearance of synovial fluid with possible cause. 6. Interpret laboratory tests on synovial fluid. 7. Suggest further testing for synovial fluid, based on preliminary results. 8. List the four classes or categories of joint disease. 9. Correlate synovial fluid analyses with their representative disease classification. 253 LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 254 Aptara Inc 254 Graff’s Textbook of Routine Urinalysis and Body Fluids oint fluid is called synovial fluid because of its resem- blance to egg white. It is a viscous, mucinous substance Jthat lubricates most joints. Analysis of synovial fluid is important in the diagnosis of joint disease. Aspiration of joint fluid is indicated for any patient with a joint effusion or inflamed joints. Aspiration of asymptomatic joints is beneficial for patients with gout and pseudogout as these fluids may still contain crystals.1 Evaluation of physical, chemical, and microscopic characteristics of synovial fluid comprise routine analysis. This chapter includes an overview of the composition and function of synovial fluid, and laboratory procedures and their interpretations. -
The Importance of Orofacial Myofunctional Therapy Before and After CO2 Laser Frenectomy in Achieving Optimal Orofacial Function
LASERfocus The Importance of Orofacial Myofunctional Therapy Before and After CO2 Laser Frenectomy in Achieving Optimal Orofacial Function by Karen M. Wuertz, DDS, ABCDSM, DABLS, FOM, and Brooke Pettus, RDH, BSDH, COMS Frenectomy Methods ing, speaking, and breathing patterns may be Frenotomies performed with a scalpel or scissors can be accompanied by caused by incorrect oral posture and oral re- significant bleeding, obscuring the surgical field making it difficult to ensure strictions. Therefore, in the authors’ opinion, if the restriction has been completely removed. Because of the increased risk the removal of oral restrictions is necessary to of early primary closure of the site, postoperative active wound care is es- attain optimal orofacial function, and must be sential to reduce the risk of potential scarring. To properly restore and main- combined with regular pre- and post-frenecto- tain optimum function, active wound care should be implemented as soon my orofacial myofunctional therapy (OMT).1,4 as possible. However, if sutures are placed, the active wound care may be OMT helps re-educate the tongue and orofa- delayed so as not to cause early tearing of tissue. Due to the contact nature of cial muscles during movement and at rest to conventional procedure, there is a certain potential for infection; in addition, create new neuromuscular patterns for proper higher levels of postoperative pain and discomfort have been reported.1,2 Elec- oral function, including chewing, swallowing, trocautery and a hot glass tip of dental diodes may leave a fairly substantial speaking, and breathing.5,6 Camacho et al.7 zone of thermal tissue change3 and may result in delayed healing.