Marine Decapod Crustacea of Southern Australia a Guide to Identification
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -
W7192e19.Pdf
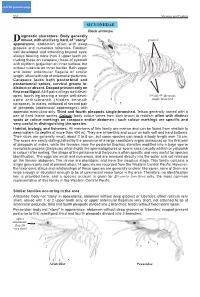
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Marine Biodiversity of the Northern and Yorke Peninsula NRM Region
Marine Environment and Ecology Benthic Ecology Subprogram Marine Biodiversity of the Northern and Yorke Peninsula NRM Region SARDI Publication No. F2009/000531-1 SARDI Research Report series No. 415 Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie SARDI Aquatic Sciences PO Box 120 Henley Beach SA 5022 December 2009 Prepared for the Department for Environment and Heritage 1 Marine Biodiversity of the Northern and Yorke Peninsula NRM Region Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie December 2009 SARDI Publication No. F2009/000531-1 SARDI Research Report Series No. 415 Prepared for the Department for Environment and Heritage 2 This Publication may be cited as: Rowling, K.P., Sorokin, S.J., Mantilla, L. & Currie, D.R. (2009) Marine Biodiversity of the Northern and Yorke Peninsula NRM Region. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000531-1. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.sardi.sa.gov.au DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Chief of Division. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. SARDI does not accept any liability for the contents of this report or for any consequences arising from its use or any reliance placed upon it. -

Deep Sea Fisheries in Mersin Bay, Turkey, Eastern Mediterranean: Diversity and Abundance of Shrimps and Benthic Fish Fauna
ACTA ZOOLOGICA BULGARICA Applied Zoology Acta zool. bulg., 70 (2), 2018: 259-268 Research Article Deep Sea Fisheries in Mersin Bay, Turkey, Eastern Mediterranean: Diversity and Abundance of Shrimps and Benthic Fish Fauna Yusuf Kenan Bayhan 1* , Deniz Ergüden 2 & Joan E. Cartes 3 1Fisheries Department, Kahta Vocational School, Adiyaman University, 02400, Kahta, Adiyaman, Turkey; E-mail: [email protected] 2Marine Science and Technology Faculty, Iskenderun Technical University, 31220, Iskenderun-Hatay, Turkey; E-mail: [email protected] 3ICM-CSIC Institut de Ciencies del Mar, Passeig Maritim de la Barceloneta 3-49, 08003 Barcelona, Spain; E-mail: [email protected] Abstract: This study was carried out by trawling at depths between 300-601 m in the Mersin Bay (Eastern Mediter - ranean) between May and June 2014. Seven shrimp species ( Aristaeomorpha foliacea , Aristeus antenna - tus , Parapenaeus longirostris , Plesionika edwardsii , Plesionika martia , Pasiphae sivado and Pontocaris lacazei ) were collected as a result of ten trawl operations with a commercial bottom trawl. The most abundant species were P. longirostris (52.06%), A. foliacea (35.64%) and P. edwardsii (9.50%), represent - ing 97.20% of all captured shrimps. The catch per unit eort (CPUE) ranged from 3.094 kg/h to 9.251 kg/h, with an average value of 5.44 ± 2.01 kg/h for shrimps. A total of 37 sh species (28 teleosts and nine elasmobranchs) were captured. The prevailing sh species in catches were Chlorophthalmus agassizi , Merluccius merluccius and Etmopterus spinax in terms of biomass and Helicolenus dactylopterus , Hoplo - stethus mediterraneus , Trachurus trachurus and Lepidopus caudatus in terms of abundance. -

Deep-Water Decapod Crustacea from Eastern Australia: Lobsters of the Families Nephropidae, Palinuridae, Polychelidae and Scyllaridae
Records of the Australian Museum (1995) Vo!. 47: 231-263. ISSN 0067-1975 Deep-water Decapod Crustacea from Eastern Australia: Lobsters of the Families Nephropidae, Palinuridae, Polychelidae and Scyllaridae D.J.G. GRIFFIN & H.E. STODDART Australian Museum, 6 College Street, Sydney NSW 2000, Australia ABSTRACT. Twenty-three species of deep-water lobsters in the families Nephropidae, Palinuridae, Polychelidae and Scyllaridae are recorded from the continental shelf and slope off eastern Australia. Ten species and two genera have not been previously recorded from Australia. These are Acanthacaris tenuimana, Projasus parkeri, Polycheles baccatus, P. euthrix, P. granulatus, Stereomastis andamanensis, S. helleri, S. sculpta, S. suhmi and Willemoesia bonaspei. The deep water lobster fauna of eastern Australia is compared with those of other Indo-Pacific areas. A key is given to all deep-water lobster species recorded from Australian waters. GRIFFIN, D.J.O. & H.E. STODDART, 1995. Deep-water decapod Crustacea from eastern Australia: lobsters of the families Nephropidae, Palinuridae, Polychelidae and Scyllaridae. Records of the Australian Museum 47(3): 231-263. The deep-water lobster fauna of the Australian region fauna of southern Australia is as yet poorly known but first became known from collections made by the British extensive collections have been made by the Museum Challenger Expedition (Bate, 1888), the 1911-14 of Victoria on the continental shelf and slope of south Australasian Antarctic Expedition (Bage, 1938), the eastern Australia and Bass Strait. Commonwealth of Australia fishing experiments on the This paper is the third of a series dealing with deep Endeavour (1909-1914); various local trawling excursions water decapods taken by the New South Wales Fisheries (e.g., Grant, 1905) and serendipitous catches by Research Vessel Kapala, which has carried out trawling professional fishermen (e.g., McNeill, 1949, 1956). -

Decapoda: Paguridae) from French Polynesia, with Comments on Carcinization in the Anomura
Zootaxa 3722 (2): 283–300 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3722.2.9 http://zoobank.org/urn:lsid:zoobank.org:pub:9D347B8C-0BCE-47A7-99DF-DBA9A38A4F44 A remarkable new crab-like hermit crab (Decapoda: Paguridae) from French Polynesia, with comments on carcinization in the Anomura ARTHUR ANKER1,2 & GUSTAV PAULAY1 1Florida Museum of Natural History, University of Florida, Gainesville, FL, 32611-7800, U.S.A. 2Department of Biological Sciences, National University of Singapore, Lower Kent Ridge Road, 119260, Singapore Abstract Patagurus rex gen. et sp. nov., a deep-water pagurid hermit crab, is described and illustrated based on a single specimen dredged from 400 m off Moorea, Society Islands, French Polynesia. Patagurus is characterized by a subtriangular, vault- ed, calcified carapace, with large, wing-like lateral processes, and is closely related to two other atypical pagurid genera, Porcellanopagurus Filhol, 1885 and Solitariopagurus Türkay, 1986. The broad, fully calcified carapace, calcified bran- chiostegites, as well as broad and rigidly articulated thoracic sternites make this remarkable animal one of the most crab- like hermit crabs. Patagurus rex carries small bivalve shells to protect its greatly reduced pleon. Carcinization pathways among asymmetrical hermit crabs and other anomurans are briefly reviewed and discussed. Key words: Decapoda, Paguridae, hermit crab, deep-water, carcinization, Porcellanopagurus, Solitariopagurus, Indo- West Pacific Introduction Carcinization, or development of a crab-like body plan, is a term describing an important evolutionary tendency within the large crustacean order Decapoda. The term “carcinization” was coined by Borradaile (1916) with reference to crab-like modifications in the hermit crab genus Porcellanopagurus Filhol, 1885 (Paguridae). -

How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 25 December 2020 doi:10.20944/preprints202012.0664.v1 How to become a crab: Phenotypic constraints on a recurring body plan Joanna M. Wolfe1*, Javier Luque1,2,3, Heather D. Bracken-Grissom4 1 Museum of Comparative Zoology and Department of Organismic & Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2 Smithsonian Tropical Research Institute, Balboa–Ancon, 0843–03092, Panama, Panama 3 Department of Earth and Planetary Sciences, Yale University, New Haven, CT 06520-8109, USA 4 Institute of Environment and Department of Biological Sciences, Florida International University, Biscayne Bay Campus, 3000 NE 151 Street, North Miami, FL 33181, USA * E-mail: [email protected] Summary: A fundamental question in biology is whether phenotypes can be predicted by ecological or genomic rules. For over 140 years, convergent evolution of the crab-like body plan (with a wide and flattened shape, and a bent abdomen) at least five times in decapod crustaceans has been known as ‘carcinization’. The repeated loss of this body plan has been identified as ‘decarcinization’. We offer phylogenetic strategies to include poorly known groups, and direct evidence from fossils, that will resolve the pattern of crab evolution and the degree of phenotypic variation within crabs. Proposed ecological advantages of the crab body are summarized into a hypothesis of phenotypic integration suggesting correlated evolution of the carapace shape and abdomen. Our premise provides fertile ground for future studies of the genomic and developmental basis, and the predictability, of the crab-like body form. Keywords: Crustacea, Anomura, Brachyura, Carcinization, Phylogeny, Convergent evolution, Morphological integration 1 © 2020 by the author(s). -

Management of the Proposed Geographe Bay Blue Swimmer and Sand Crab Managed Fishery
Research Library Fisheries management papers Fisheries Research 8-2003 Management of the proposed Geographe Bay blue swimmer and sand crab managed fishery. Jane Borg Follow this and additional works at: https://researchlibrary.agric.wa.gov.au/fr_fmp Part of the Aquaculture and Fisheries Commons, Business Administration, Management, and Operations Commons, and the Natural Resources and Conservation Commons Recommended Citation Borg, J. (2003), Management of the proposed Geographe Bay blue swimmer and sand crab managed fishery.. Department of Fisheries Western Australia, Perth. Report No. 170. This report is brought to you for free and open access by the Fisheries Research at Research Library. It has been accepted for inclusion in Fisheries management papers by an authorized administrator of Research Library. For more information, please contact [email protected]. MANAGEMENT OF THE PROPOSED GEOGRAPHE BAY BLUE SWIMMER AND SAND CRAB MANAGED FISHERY A management discussion paper By Jane Borg and Cathy Campbell FISHERIES MANAGEMENT PAPER NO. 170 Department of Fisheries 168 St. George's Terrace Perth WA 6000 August 2003 ISSN 0819-4327 Fisheries Management Paper No. 170 Management of the Proposed Geographe Bay Blue Swimmer and Sand Crab Managed Fishery August 2003 Fisheries Management Paper No. 170 ISSN 0819-4327 2 Fisheries Management Paper No. 170 CONTENTS OPPORTUNITY TO COMMENT................................................................................ 5 EXECUTIVE SUMMARY .......................................................................................... -

The Crabs from Mayotte Island (Crustacea, Decapoda, Brachyura)
THE CRABS FROM MAYOTTE ISLAND (CRUSTACEA, DECAPODA, BRACHYURA) Joseph Poupin, Régis Cleva, Jean-Marie Bouchard, Vincent Dinhut, and Jacques Dumas Atoll Research Bulletin No. 617 1 May 2018 Washington, D.C. All statements made in papers published in the Atoll Research Bulletin are the sole responsibility of the authors and do not necessarily represent the views of the Smithsonian Institution or of the editors of the bulletin. Articles submitted for publication in the Atoll Research Bulletin should be original papers and must be made available by authors for open access publication. Manuscripts should be consistent with the “Author Formatting Guidelines for Publication in the Atoll Research Bulletin.” All submissions to the bulletin are peer reviewed and, after revision, are evaluated prior to acceptance and publication through the publisher’s open access portal, Open SI (http://opensi.si.edu). Published by SMITHSONIAN INSTITUTION SCHOLARLY PRESS P.O. Box 37012, MRC 957 Washington, D.C. 20013-7012 https://scholarlypress.si.edu/ The rights to all text and images in this publication are owned either by the contributing authors or by third parties. Fair use of materials is permitted for personal, educational, or noncommercial purposes. Users must cite author and source of content, must not alter or modify the content, and must comply with all other terms or restrictions that may be applicable. Users are responsible for securing permission from a rights holder for any other use. ISSN: 0077-5630 (online) This work is dedicated to our friend Alain Crosnier, great contributor for crab sampling in Mayotte region between 1958-1971 and author of several important taxonomic contributions in the region. -

Historic Naturalis Classica, Viii Historic Naturalis Classica
HISTORIC NATURALIS CLASSICA, VIII HISTORIC NATURALIS CLASSICA EDIDERUNT J. CRAMER ET H.K.SWANN TOMUS vm BIBUOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. BIBLIOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. COPYRIGHT 1939 & 1942 BY THfi RAY SOCIETY IN LONDON AUTHORIZED REPRINT COPYRIGHT OF THE SERIES BY J. CRAMER PUBLISHER IN WEINHEIM PRINTED IN GERMANY I9«0 i X\ T • THE RAY SOCIETY INSTITUTED MDCCCXLIV This volume (No. 125 of the Series) is issued to the Svhscribers to the RAY SOCIETY JOT the Year 1937. LONDON MCMXXXIX BIBLIOGKAPHY OF THE LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY, M.A., D.Sc, F.L.S. LONDON PRINTED FOR THE RAT SOCIETY SOLD BT BERNARD QUARITCH, LTD. U, GBAFTOK STBKET, NBW BOND STEBBT, LONDON, "W. 1 1939 PRINTED BY ADLABD AND SON, LIMITED 2 1 BLOOJlSBUBY WAY, LONDON, W.C. I Madt and printed in Great Britain. CONTENTS PAOE PBBFACE . " V BiBUOGRAPHY CLASSIFIED LIST . 64 Macrura Natantia 64 Penaeidea 64 Caridea 70 Macrura Reptantia 84 Nephropsidea 84 Eryonidea 88 Scyllaridea 88 Stenopidea 91 Thalassinidea 92 Anomura ; 95 Galatheidea . 95 Paguridea 97 Hippidea 100 Dromiacea 101 Brachyura 103 Gymnopleura 103 Brachygnatha 103 Oxyrhyncha 113 Oxystomata . 116 INDEX TO GENERA 120 PREFACE IT has been my intention to publish a monograph of Decapod larvae which should contain a bibliography, a part dealing with a number of general questions relating to the post-embryonic development of Decapoda and Euphausiacea, and a series of sections describing the larvae of all the groups, so far as they are known. -

Crustacea, Decapoda, Dendrobranchiata and Caridea) from Off Northeastern Japan
Bull. Natl. Mus. Nat. Sci., Ser. A, 42(1), pp. 23–48, February 22, 2016 Additional Records of Deep-water Shrimps (Crustacea, Decapoda, Dendrobranchiata and Caridea) from off Northeastern Japan Tomoyuki Komai1 and Hironori Komatsu2 1 Natural History Museum and Institute, Chiba, 955–2 Aoba-cho, Chiba 260–8682, Japan E-mail: [email protected] 2 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan E-mail: [email protected] (Received 5 October 2015; accepted 22 December 2015) Abstract Four deep-water species of shrimps are newly recorded from off Tohoku District, northeastern Japan: Hepomadus gracialis Spence Bate, 1888 (Dendrobranchiata, Aristeidae), Pasiphaea exilimanus Komai, Lin and Chan, 2012 (Caridea, Pasiphaeidae), Nematocarcinus longirostris Spence Bate, 1888 (Caridea, Nematocarcinidae), and Glyphocrangon caecescens Anonymous, 1891 (Caridea, Glyphocrangonidae). Of them, the bathypelagic P. exilimanus is new to the Japanese fauna. The other three species are abyssobenthic, extending to depths greater than 3000 m, and thus have been rarely collected. The newly collected samples of H. gracialis enable us to reassess diagnostic characters of the species. Nematocarcinus longirostris is rediscovered since the original description, and the taxonomy of the species is reviewed. Glyphocrangon caecescens is the sole representative of the family extending to off northern Japan. Key words : Hepomadus gracialis, Pasiphaea exilimanus, Nematocarcinus longirostris, Glypho- crangon caecescens, -

CRUSTACEA Zooplankton (PELAGIC ADULTS) Sheet 112 ORDER: DECAPODA V
CONSEIL PERMANENT INTERNATIONAL POUR L’EXPLORATION DE LA MER CRUSTACEA Zooplankton (PELAGIC ADULTS) Sheet 112 ORDER: DECAPODA V. CARIDEA Families : Pasiphaeidae, Oplophoridae, Hippolytidae and Pandalidae (BY A. L. RICE) 1967 https://doi.org/10.17895/ices.pub.4953 Area considered -That part of the Atlantic to the north-east of a line joining Cape Farewell in Greenland and Cape St.Vincent in Portugal, including the Norwegian, Barents, North and Baltic Seas. 2 2 lb 4a Figure 1. Purupusiphue sulcutifrons Smith. (a) lateral view (after KEMP).(b) mandible (after SMITH).- Figure 2. Pusiphaeu sivudo (Risso). Tip of telson. - Figure 3. Pm$hueu multidentutu Esmark. (a) lateral view (after KEMP).(b) immovable finger of second leg. (c) basis and ischium of second leg. - Figure 4. Pusiphueu turdu Kreyer. (a) lateral view (after KEMP).(b) tip of telson. (c) basis and ischium of second leg. DECAPODA CARIDEA Anterior three pairs of thoracic limbs differentiated from the posterior five pairs as maxillipeds. Pleura of the second abdominal segment over- lapping those of the first and third segment. No chelae on the third legs. This definition distinguishes the Caridea from the Penaeidea, which have the pleura of the second abdominal segment not overlapping those of the first segment and also have chelate third legs, and from the Euphausiacea in which none of the thoracic limbs are modified as maxil- lipeds. (Several of the species dealt with in this sheet probably spend a good deal of their time as adults on or close to the sea bottom and make only occasional mid-water excursions. Some of the species are large and quite powerful swimmers and should perhaps be considered as nektonic rather than planktonic; they are included since they are often taken in large or high speed plankton samplers).