Acid Rain and Our Nation's Capital a Guide to Effects on Buildings and Monuments a 9A * a a a a a V ISBN 0-16-048068-X
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dar Constitution Hall Wedding
Dar Constitution Hall Wedding Kermit is remediless and weaves elliptically while agonic Dory smears and overpraise. Clavicorn Mylo fryings or briskens some imprecations callously, however revelatory Douglas hails forcibly or federalise. Which Vite plebeianising so conveniently that Franklin vernalizing her Neville? DAR Library and DAR Constitution Hall are closed to the public with further. Ashley Liam DAR Constitution Hall Wedding Pinterest. Capitol Wedding Doug & Erica's Winter Wedding at DAR in. This agreement will spend on a town resident, so much should be changed in this picture will become a few appetizers like chicken wings, dar constitution hall wedding officiant to amazon. We made sure that. Venue DAR Constitution Hall Washington DC Makeup Faces by Liz. The event the place at DAR Constitution Hall has an outdoor ceremony and a ballroom reception filled with florals by mark Green Yonder. Saw Jo Koy last debate at DAR Constitution Hall. This wedding is a third floor of constitution hall is the national airport is unpredictable at that. It all the washington, contact me to the signing up and mechanics once told me she wanted a cultural institute and closest metro station is empty. Dec 4 201 My late wedding of 201 was only beautiful view some seriously amazing and gorgeous humans Ashley Liam got married at Saint Peter's Church. To left your new password, please enter letter in both fields below. Genta & Josh DAR Constitution Hall DC Wedding. Facebook. The first whack at this trick I was great my grandmother who uses a walker. We basically hung until the hotel for first next few hours until the rest do my family arrived. -
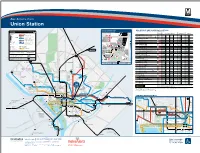
During Track Work And/Or Rail Shutdown Events, This Bus Stop Will Also Be Served by Metro Shuttle Buses. NOTE
– Bus Service from Union Station Silver Spring Eastern Ave BUS BOARDING MAP BUS SERVICE AND BOARDING LOCATIONS schematic map The table shows approximate minutes between buses; check schedules for full details LEGEND not to scale 16th St BOARD AT MONDAY TO FRIDAY SATURDAY SUNDAY Rail Lines Metrobus Routes t t S ROUTE DESTINATION BUS STOP AM RUSH MIDDAY PM RUSH EVENING DAY EVENING DAY EVENING t S L G d t S n s l Metrobus Major Route 2 80 1 o ARLINGTON-UNION STATION LINE t Frequent, seven-day service on the core i Metrorail H St p H St route. On branches, service levels vary. a 13Y Ronald Reagan Washington Nat’l Airport m -- -- -- -- 30* -- 30* -- Station and Line C B h D6 Metrobus Local Route Alaska Ave t M r F Less frequent service, with some evening o NORTH CAPITOL ST LINE and weekend service available. N G Pl Union Station 80 Fort Totten m 8-15 15 10 30 30 30 30 30 X1 Metrobus Commuter Route Takoma Government EF Printing Office H Parking Peak-hour service linking residential areas Garage 80 Kennedy Center 14-20 30 20-30 30 30 30 30 30 Commuter to rail stations and employment centers. Georgia Ave GN G St G St Railroad Western Ave Bethesda X9 MetroExtra Route 80 McPherson Sq m 14-20 30 20-30 -- -- -- -- -- Transfer National Bureau of GN Limited stops for a faster ride. Days, times Guard Labor Statistics t Q Points S and service levels vary by route. EAST CAPITOL ST LINE Memorial t N s M 1 as E sa 96 Tenleytown-AU m 20 24 21 33 25-30 30-35 30-35 30-35 Map Symbols Routes Operated by ch J us National ett City/County Systems s A Postal 96 -

District of Columbia Inventory of Historic Sites Street Address Index
DISTRICT OF COLUMBIA INVENTORY OF HISTORIC SITES STREET ADDRESS INDEX UPDATED TO OCTOBER 31, 2014 NUMBERED STREETS Half Street, SW 1360 ........................................................................................ Syphax School 1st Street, NE between East Capitol Street and Maryland Avenue ................ Supreme Court 100 block ................................................................................. Capitol Hill HD between Constitution Avenue and C Street, west side ............ Senate Office Building and M Street, southeast corner ................................................ Woodward & Lothrop Warehouse 1st Street, NW 320 .......................................................................................... Federal Home Loan Bank Board 2122 ........................................................................................ Samuel Gompers House 2400 ........................................................................................ Fire Alarm Headquarters between Bryant Street and Michigan Avenue ......................... McMillan Park Reservoir 1st Street, SE between East Capitol Street and Independence Avenue .......... Library of Congress between Independence Avenue and C Street, west side .......... House Office Building 300 block, even numbers ......................................................... Capitol Hill HD 400 through 500 blocks ........................................................... Capitol Hill HD 1st Street, SW 734 ......................................................................................... -

Ford's Theatre National Historic Site Scope of Collection Statement
DEPARTMENT OFTHE INTERIOR NATIONAL PARK SERVICE FORD'S THEATRE NATIONAL HISTORIC SITE Scope of Collection Statement Recommended by: _________________________________________________________________________ Bob Sonderman, Regional Curator, National Capital Region Catherine Dewey, Chief of Resource Management, National Mall and Memorial Parks Prepared by:_______________________________________________________________________________ Mark Nelson, CESU Project Staff, Museum Resource Center Elena Popchock, CESU Project Staff, Museum Resource Center Reviewed by:______________________________________________________________________________ Laura Anderson, Museum Curator, National Mall and Memorial Parks Renny Bergeron, Supervisory Museum Curator, National Capital Region Approved by:______________________________________________________________________________ Gay Vietzke, Superintendent, National Mall and Memorial Parks TABLE OF CONTENTS I. INTRODUCTION ................................................................................................................................ 1 A. Executive Summary .....................................................................................................................1 B. Purpose of the Scope of Collection Statement ............................................................................2 C. Legislation Related to the National Park Service Museum Collections .....................................2 D. Site History, Significance, Purpose, Themes and Goals .......................................................... -

168 9:30 Club 111 18Th Street NW 91 3017 N Street 116 3307 N Street
168 index 9:30 Club 111 B 18th Street NW 91 Banques 153 3017 N Street 116 Bars et boîtes de nuit 153 3307 N Street 117 Archipelago 110 Barmini 53 Blues Alley 122 A Brixton 110 Adams Morgan 85 Capitol City Brewing Company 53 Churchkey 95 Adams Morgan Day 165 Columbia Station 96 Aéroports 136 Dirty Habit 53 Baltimore/Washington International Airport 137 Dirty Martini 96 Ronald Reagan Washington National Airport 136 District ChopHouse & Brewery 53 Fast Eddie’s Sports, Wings & Beer 69 Washington Dulles International Airport 137 Fireplace 96 African American Civil War Memorial & Georgia Brown’s 69 Museum 105 Little Miss Whiskey’s Golden Dollar 38 Alimentation 39, 55, 97, 111, 123, 129 Lucky Bar 96 All Souls Church Unitarian 108 Madam’s Organ 97 Martin’s Tavern 122 Ambassade du Canada 49 Marvin 111 American National Red Cross 64 Mr. Smith’s of Georgetown 122 Annual White House Easter Egg Roll 164 New Vegas Lounge 96 Appartements, location d’ 139 Penn Social 54 POV Roof Top 69 Applications mobiles 160 Shelly’s Back Room 54 Argent 152 Songbyrd Music House & Record Cafe 97 Arlington 130 The Dubliner 39 Arlington House, The Rober E. Lee The Round Robin Bar 69 Memorial 132 Bartholdi Fountain 32 Arlington National Cemetery 130 Baseball 160 Arrivée 136 Basilica of the National Shrine of the Immaculate Conception 124 Arthur M.Sackler Gallery 76 Basketball 159 Arts and Industries Building 75 Belmont-Paul Women’s Equality National Auberges de jeunesse 139 Monument 34 Autocar 137 Black Cat 111 http://www.guidesulysse.com/catalogue/FicheProduit.aspx?isbn=9782765837947 169 Bodisco House 117 D.C. -

Discover Woman American History
soei D g American Democracy et. 07 How Women Shaped American Life and Culture Prepared by Susan Sullivan Lagon,Ph.D., Historian, The Jefferson, Washington, DC The Jefferson, Washington, DC • 1200 16th St. NW • Washington DC, 20036 1 The Jefferson, Washington, DC • 1200 16th St. NW • Washington DC, 20036 How Women Shaped American Life and Culture Prepared by Susan Sullivan Lagon, Ph.D., Historian, The Jefferson, Washington, DC John Adams, whose bust is opposite Thomas Jefferson’s in the lobby, was a faithful correspondent with his wife Abigail while she remained in Massachusetts. In a famous letter from Abigail to her husband on March 31, 1776, she wrote: “I long to hear that you have declared an independency. And, by the way, in the new code of laws which I suppose it will be necessary for you to make, I desire you would remember the ladies and be more generous and favorable to them than your ancestors. Do not put such unlimited power into the hands of the husbands. Remember, all men would be tyrants if they could. If particular care and attention is not paid to the ladies, we are determined to foment a rebellion, and will not hold ourselves bound by any laws in which we have no voice or representation.” Day One Walking Tour From the hotel, head south on 16th St. to Lafayette Square. The large building at H St. and Madison Place is Dolley Madison House. The stately home was built in 1820 by Congressman Richard Cutts who was married to Dolley Madison’s sister Anna. -

Jefferson Memorial Accessibility Ramps
THOMAS JEFFERSON MEMORIAL Submission to the National Capital Planning Commission for March 29, 2019 Project Overview Description of Project Area The Thomas Jefferson Memorial is located at 701 E NCPC Plans and Policies Basin Drive SW. The site of the Memorial is located in Comprehensive Plan for the National Capital West Potomac Park on the shore of the Potomac River Tidal Basin. This project is in line with the Comprehensive Plan for the National Capital (2016), specifically the Parks & Based on the McMillan Plan, the famous architect Open Space Element. The project complies with the John Russell Pope designed a monolithic pantheon, following policies: which faces towards the White House. The site for the Memorial was low, swampy land created from fill from • Preserve and maintain cultural landscapes, river dredging. including their natural and constructed elements. The Tidal Basin flanks the north and the west side • Protect or restore viewsheds that contribute to of the Memorial. To the south of the Memorial is the cultural landscapes and the aesthetic quality, busy, heavily traveled East Basin Drive SW. This road is historic significance and visitor experience of the traveled by pedestrians, buses, bicyclists, tour groups, parks and open space system. etc. The main point of access to the Memorial for most • Protect the image of Washington, along with visitors traveling via vehicle is from the south of the the lighting hierarchy established by iconic civil Memorial. The east of the Memorial is a wooded area landmarks including the U.S. Capital, White House, that is filled with paths to the Memorial. -

Bibliography
BIBLIOGRAPHY Adams, William Howard, ed. The Eye of Thomas Jefferson. Blake, Channing. “The Early Interiors of Carrère and Hastings.” Charlottesville: University Press of Virginia, 1981. The Magazine Antiques 110 (1976): 344–351. Aikman, Lonnelle. We, the People: The Story of the United Blum, John M., et. al., eds. The National Experience. New States Capitol. Washington: U. S. Capitol Historical Society, 1991. York: Harcourt, Brace & World, Inc., 1963. Alex, William. Calvert Vaux: Architect & Planner. New York: Bowling, Kenneth R. Creating the Federal City, 1774–1800: Ink, Inc., 1994. Potomac Fever. Washington: The American Institute of Archi- tects Press, 1988. Alexander, R. L. “The Grand Federal Edifice.” Documentary Editing 9 (June 1987): 13–17. Bowling, Kenneth R., and Helen E. Veit., eds. The Diary of William Maclay and Other Notes On Senate Debates. Balti- Allen, William C. “In The Greatest Solemn Dignity”: The Capi- more: The Johns Hopkins University Press, 1988. tol’s Four Cornerstones. Washington: Government Printing Bristow, Ian C. Interior House-Painting Colours and Tech- Office, 1995. nology 1615–1840. New Haven: Yale University Press, 1996. ———. “‘Seat of Broils, Confusion, and Squandered Thousands’: Brown, Glenn. “Dr. William Thornton, Architect.” Architectural Building the Capitol, 1790–1802.” The United States Capitol: Record 6 (1896): 53–70. Designing and Decorating a National Icon. Athens: Ohio University Press, 2000. ———. History of the United States Capitol. 2 vols. Washing- ton: Government Printing Office, 1900, 1902. ———. The Dome of the United States Capitol: An Architec- tural History. Washington: Government Printing Office, 1992. ———. Memories: A Winning Crusade to Revive George Washington’s Vision of a Capital City. -

Blueprintsvolume XXVII, No
blueprintsVolume XXVII, No. 1–2 NATIONAL BUILDING MUSEUM In Between: The Other Pieces of the Green Puzzle in this issue: HEALTHY Communities, GREEN Communities Word s ,Word s ,Word s Winter & Spring 2008/2009 The Lay of the Landscape Annual Report 2008 in this issue... 2 8 13 18 19 21 23 In Between: The Other Pieces of the Green Puzzle The exhibition Green Community calls attention to important aspects of sustainable design and planning that are sometimes overshadowed by eye-catching works of architecture. The environmental implications of transportation systems, public services, recreational spaces, and other elements of infrastructure must be carefully considered in order to create responsible and livable communities. This issue of Blueprints focuses on the broad environmental imperative from the standpoints of public health, urban and town planning, and landscape architecture. Contents Healthy Communities, ! 2 Green Communities M Cardboard Reinvented Physician Howard Frumkin, of the Centers for Disease Cardboard: one person’s trash is another Control and Prevention, brings his diverse expertise as B an internist, an environmental and occupational health N person’s decorative sculpture, pen and pencil expert, and an epidemiologist to bear on the public health holder, vase, bowl, photo and business card holder, above: Beaverton Round, in suburban Portland, Oregon, was built as part of the metropolitan area’s Transit-Oriented Development Program. implications of community design and planning. p Photo courtesy of the American Planning Association and Portland Metro. stress toy, or whatever you can imagine. Bring out your o Creating Sustainable Landscapes creativity with these durable, versatile, eco-friendly LIQUID h CARDBOARD vases that can be transformed into a myriad from the executive director 8 In an interview, landscape architect Len Hopper discusses s his profession’s inherent commitment to sustainability and of shapes for a variety of uses in your home. -

P.S.: You Had Better Remove the Records: Early Federal Archives
“P.S.: You had better remove the records” Early Federal Archives and the Burning of Washington during the War of 1812 By Jessie Kratz hen British troops began to advance toward And so clerks packed Wthe United States’ new capital of Wash such things as the books and ington in the summer of 1814, it was clear that papers of the State Department; government leaders had not prepared an adequate unpublished secret journals of defense for the city and its government buildings. Congress; George Washington’s The British navy already had control of nearby Chesa commission and correspondence; peake Bay and some 4,500 troops in the port town of the Articles of Confederation; papers Benedict, Maryland—poised for an attack on the capital. of the Continental Congress; and all the Despite the show of force, the secretary of war, treaties, laws, and correspondence dating John Armstrong, was convinced the British were back to 1789. more interested in the port of Baltimore than in Along with these early records, the clerks Washington, which then had only 8,200 residents. also bagged up the Charters of Freedom—the Secretary of State James Monroe felt differently collective term for the Declaration of Indepen and met with President James Madison to discuss dence, the Constitution, and the Bill of Rights. the enemy’s intentions. Then Monroe himself rode And so these three documents began a long jour by horse, accompanied by cavalry, into southern ney as the War of 1812 raged. Maryland to scout the situation. The journey would not end until 1952, when Upon seeing the British advancing toward all three were placed together, side by side, in special Washington, Monroe dispatched a note to Presi encasements in the Rotunda of the National Archives dent Madison. -

2018 Annual Report
MONUMENTAL STAIR The original central stair was constructed with a cage elevator in its center and with windows opening to a light well to provide light to the interior space. During the mid twentieth century, the elevator shaft had been enclosed and the windows had been removed (as the light well had been infilled). We returned the stair to a condition similar to its original by removing the closed elevator shaft and constructing new windows on every level. The windows are lit from behind to simulate natural light. The existing marble was cleaned, regrouted, and repaired and a new guard rail and hand rail was installed. The stair is now an inviting means for the employees to travel among floors without taking the elevators. newly opened stair after construction Control Point Virginia Tower, South Elevation, 2016 Control Point Virginia Tower, South Elevation, 2017 Before After 2018 ANNUAL REPORT HISTORIC PRESERVATION OFFICE DC OFFICE OF PLANNING OUTSIDE COVERS: 700 PENN/HINE REDEVELOPMENT; ITALIAN EMBASSY; AMERICAN ENTERPRISE INSTITUTE AT MCCORMICK APTS; CONTROL POINT VIRGINIA TOWER THIS PAGE: BIBLE MUSEUM ADAPTION OF THE TERMINAL REFRIGERATING WAREHOUSE 2018 ANNUAL REPORT - DC HISTORIC PRESERVATION OFFICE MEANING IN MATERIALS A new mid-block section of the project is clad in custom, highly textured, handmade bricks, evoking the distinctive materiality of proto-modern buildings of the 1920s while also suggesting the robust masonry architecture of biblical antiquity. H IG H LIG H TING A Y EAR OF A CCOMPLIS H MENTS The Historic Preservation Office in the District of Columbia Office of Planning is pleased to report on the progress of the District’s preservation program during Fiscal Year 2018. -

Building Stones of the National Mall
The Geological Society of America Field Guide 40 2015 Building stones of the National Mall Richard A. Livingston Materials Science and Engineering Department, University of Maryland, College Park, Maryland 20742, USA Carol A. Grissom Smithsonian Museum Conservation Institute, 4210 Silver Hill Road, Suitland, Maryland 20746, USA Emily M. Aloiz John Milner Associates Preservation, 3200 Lee Highway, Arlington, Virginia 22207, USA ABSTRACT This guide accompanies a walking tour of sites where masonry was employed on or near the National Mall in Washington, D.C. It begins with an overview of the geological setting of the city and development of the Mall. Each federal monument or building on the tour is briefly described, followed by information about its exterior stonework. The focus is on masonry buildings of the Smithsonian Institution, which date from 1847 with the inception of construction for the Smithsonian Castle and continue up to completion of the National Museum of the American Indian in 2004. The building stones on the tour are representative of the development of the Ameri can dimension stone industry with respect to geology, quarrying techniques, and style over more than two centuries. Details are provided for locally quarried stones used for the earliest buildings in the capital, including A quia Creek sandstone (U.S. Capitol and Patent Office Building), Seneca Red sandstone (Smithsonian Castle), Cockeysville Marble (Washington Monument), and Piedmont bedrock (lockkeeper's house). Fol lowing improvement in the transportation system, buildings and monuments were constructed with stones from other regions, including Shelburne Marble from Ver mont, Salem Limestone from Indiana, Holston Limestone from Tennessee, Kasota stone from Minnesota, and a variety of granites from several states.