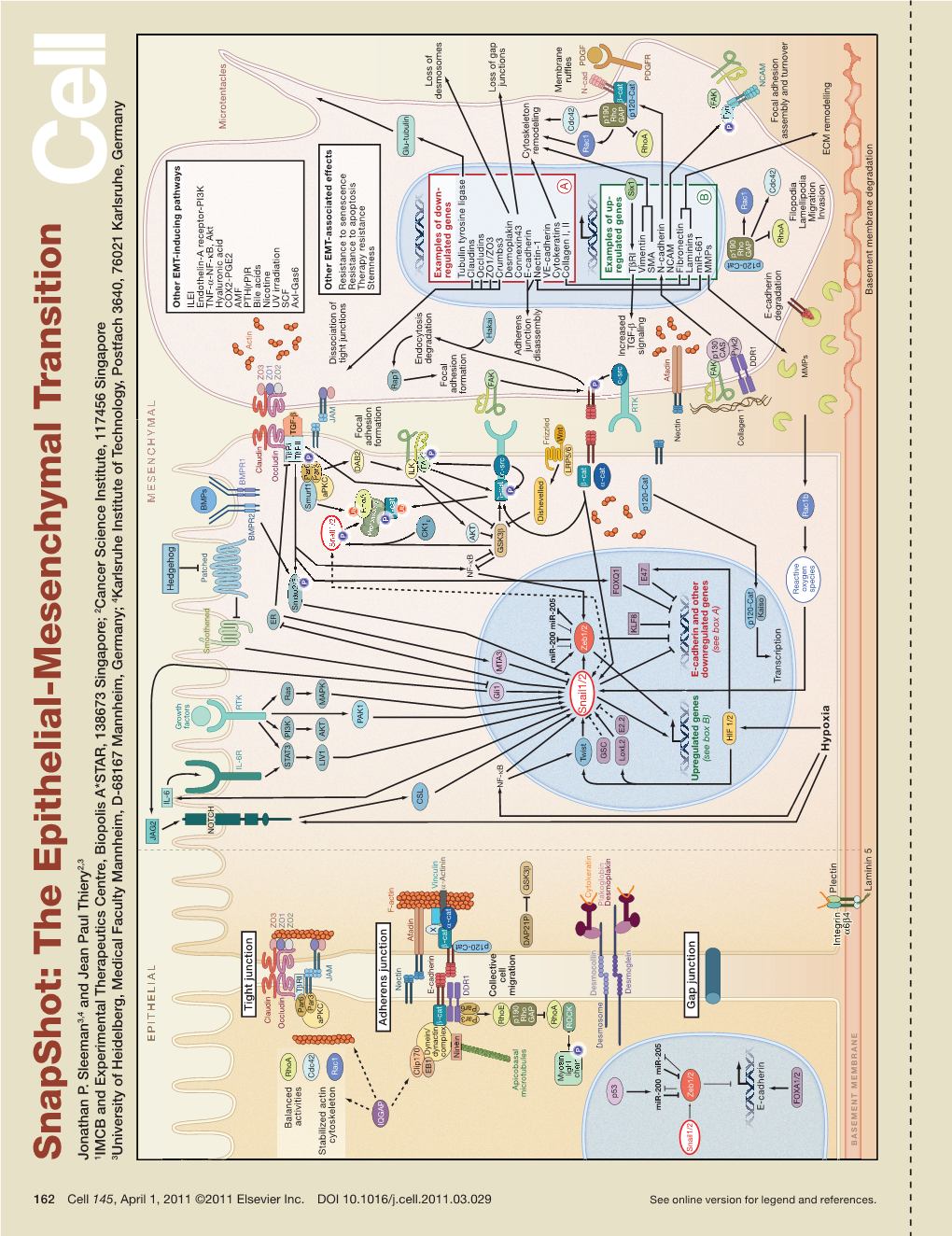
Snapshot: the Epithelial-Mesenchymal Transition Snapshot: Epithelial-Mesenchymal the Sleeman Jonathan P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARVC-Variants.Pdf
Updated gene list responsible for ARVC/D pathology Subtype Gene Location Reference ARVC1 TGFB3 14q24.3 Beffagna G, Occhi G, Nava A, et al. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res. 2005;65:366–73. ARVC2 RYR2 1q43 Tiso N, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet. 2001;10:189–94. ARVC3 Unknown 14q12-q22 Severini GM, Krajinovic M, Pinamonti B, et al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics. 1996;31:193–200. ARVC4 TTN 2q32.1-q32.3 Taylor M, Graw S, Sinagra G, et al. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–85. ARVC5 TMEM43 3p25.1 Merner ND, Hodgkinson KA, Haywood AF, et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet. 2008;82:809–21. ARVC6 Unknown 10p14-p12 Li D, Ahmad F, Gardner MJ, et al. The locus of a novel gene responsible for arrhythmogenic right- ventricular dysplasia characterized by early onset and high penetrance maps to chromosome 10p12-p14. Am J Hum Genet. 2000;66:148–56. ARVC7 DES 2q35 Klauke B, Kossmann S, Gaertner A, et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum Mol Genet. 2010;19:4595–607. -

Claudin-2: Roles Beyond Permeability Functions
International Journal of Molecular Sciences Review Claudin-2: Roles beyond Permeability Functions Shruthi Venugopal, Shaista Anwer and Katalin Szászi * Keenan Research Centre for Biomedical Science of the St. Michael’s Hospital and Department of Surgery, University of Toronto, Toronto, ON M5B 1W8, Canada; [email protected] (S.V.); [email protected] (S.A.) * Correspondence: [email protected]; Tel.: +1-416-8471752 Received: 13 October 2019; Accepted: 9 November 2019; Published: 12 November 2019 Abstract: Claudin-2 is expressed in the tight junctions of leaky epithelia, where it forms cation-selective and water permeable paracellular channels. Its abundance is under fine control by a complex signaling network that affects both its synthesis and turnover in response to various environmental inputs. Claudin-2 expression is dysregulated in many pathologies including cancer, inflammation, and fibrosis. Claudin-2 has a key role in energy-efficient ion and water transport in the proximal tubules of the kidneys and in the gut. Importantly, strong evidence now also supports a role for this protein as a modulator of vital cellular events relevant to diseases. Signaling pathways that are overactivated in diseases can alter claudin-2 expression, and a good correlation exists between disease stage and claudin-2 abundance. Further, loss- and gain-of-function studies showed that primary changes in claudin-2 expression impact vital cellular processes such as proliferation, migration, and cell fate determination. These effects appear to be mediated by alterations in key signaling pathways. The specific mechanisms linking claudin-2 to these changes remain poorly understood, but adapters binding to the intracellular portion of claudin-2 may play a key role. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Computational Modeling of Claudin Structure and Function
International Journal of Molecular Sciences Review Computational Modeling of Claudin Structure and Function Shadi Fuladi 1, Ridaka-Wal Jannat 1 , Le Shen 2,3 , Christopher R. Weber 2,* and Fatemeh Khalili-Araghi 1,* 1 Department of Physics, University of Illinois at Chicago, Chicago, IL 60607, USA; [email protected] (S.F.); [email protected] (R.-W.J.) 2 Department of Pathology, University of Chicago, Chicago, IL 60637, USA; [email protected] 3 Department of Surgery, University of Chicago, Chicago, IL 60637, USA * Correspondence: [email protected] (C.R.W.); [email protected] (F.K.-A.) Received: 15 December 2019; Accepted: 16 January 2020; Published: 23 January 2020 Abstract: Tight junctions form a barrier to control passive transport of ions and small molecules across epithelia and endothelia. In addition to forming a barrier, some of claudins control transport properties of tight junctions by forming charge- and size-selective ion channels. It has been suggested claudin monomers can form or incorporate into tight junction strands to form channels. Resolving the crystallographic structure of several claudins in recent years has provided an opportunity to examine structural basis of claudins in tight junctions. Computational and theoretical modeling relying on atomic description of the pore have contributed significantly to our understanding of claudin pores and paracellular transport. In this paper, we review recent computational and mathematical modeling of claudin barrier function. We focus on dynamic modeling of global epithelial barrier function as a function of claudin pores and molecular dynamics studies of claudins leading to a functional model of claudin channels. Keywords: claudin; molecular dynamics; tight junction; ion transport; ion channel 1. -

Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease Aaron Buckley and Jerrold R. Turner Departments of Pathology and Medicine (Gastroenterology), Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts 02115 Correspondence: [email protected] Mucosal surfaces are lined by epithelial cells. In the intestine, the epithelium establishes a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing intrusion by luminal materials. Intestinal epithelia therefore play a central role in regulating interactions between the mucosal immune system and luminal contents, which include dietary antigens, a diverse intestinal microbiome, and pathogens. The paracellular space is sealed by the tight junction, which is maintained by a complex network of protein interactions. Tight junction dysfunction has been linked to a variety of local and systemic diseases. Two molecularly and biophysically distinct pathways across the intestinal tight junc- tion are selectively and differentially regulated by inflammatory stimuli. This review discusses the mechanisms underlying these events, their impact on disease, and the potential of using these as paradigms for development of tight junction-targeted therapeutic interventions. ucosal surfaces and the epithelial cells that adherens). The tight junction is a selectively Mline them are present at sites where tissues permeable barrier that generally represents the interface directly with the external environment rate-limiting step of paracellular transport. The or internal compartments that are contiguous adherens junction and desmosome provide es- with the external environment. Examples in- sential adhesive and mechanical properties that clude the gastrointestinal tract, the pulmonary contribute to barrier function but do not seal tree, and the genitourinary tract. -

Quinone Oxidoreductase-1 in the Tight Junctions of Colonic Epithelial Cells
BMB Rep. 2014; 47(9): 494-499 BMB www.bmbreports.org Reports Role of NADH: quinone oxidoreductase-1 in the tight junctions of colonic epithelial cells Seung Taek Nam1,#, Jung Hwan Hwang2,#, Dae Hong Kim1, Mi Jung Park1, Ik Hwan Lee1, Hyo Jung Nam1, Jin Ku Kang1, Sung Kuk Kim1, Jae Sam Hwang3, Hyo Kyun Chung4, Minho Shong4, Chul-Ho Lee2,* & Ho Kim1,* 1Department of Life Science, College of Natural Science, Daejin University, Pocheon 487-711, 2Laboratory Animal Resource Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon 305-806, 3Department of Agricultural Biology, National Academy of Agricultural Science, RDA, Suwon 441-707, 4Department of Internal Medicine, Chungnam National University, Daejon 301-721, Korea NADH:quinone oxidoreductase 1 (NQO1) is known to be lating the intracellular ratio of NAD and NADH (two funda- involved in the regulation of energy synthesis and metabolism, mental mediators of energy metabolism) in various cell and the functional studies of NQO1 have largely focused on systems. NQO1 is also known as an antioxidant flavoprotein metabolic disorders. Here, we show for the first time that that scavenges reactive oxygen species (ROS) (3). Having pre- compared to NQO1-WT mice, NQO1-KO mice exhibited a viously shown that NQO1 activity is associated with cancer (4) marked increase of permeability and spontaneous inflammation and metabolic disorders, including diabetes and obesity (5), in the gut. In the DSS-induced colitis model, NQO1-KO mice we herein focused on the possible role of NQO1 in the gastro- showed more severe inflammatory responses than NQO1-WT intestinal tract. We report for the first time that the expression mice. -

Defenders and Challengers of Endothelial Barrier Function
REVIEW published: 18 December 2017 doi: 10.3389/fimmu.2017.01847 Defenders and Challengers of Endothelial Barrier Function Nader Rahimi* Department of Pathology, Boston University School of Medicine, Boston, MA, United States Regulated vascular permeability is an essential feature of normal physiology and its dysfunction is associated with major human diseases ranging from cancer to inflam- mation and ischemic heart diseases. Integrity of endothelial cells also play a prominent role in the outcome of surgical procedures and organ transplant. Endothelial barrier function and integrity are regulated by a plethora of highly specialized transmembrane receptors, including claudin family proteins, occludin, junctional adhesion molecules (JAMs), vascular endothelial (VE)-cadherin, and the newly identified immunoglobulin (Ig) and proline-rich receptor-1 (IGPR-1) through various distinct mechanisms and signaling. On the other hand, vascular endothelial growth factor (VEGF) and its tyrosine kinase receptor, VEGF receptor-2, play a central role in the destabilization of endothelial barrier function. While claudins and occludin regulate cell–cell junction via recruitment of zonula occludens (ZO), cadherins via catenin proteins, and JAMs via ZO and afadin, IGPR-1 recruits bullous pemphigoid antigen 1 [also called dystonin (DST) and SH3 protein inter- Edited by: acting with Nck90/WISH (SH3 protein interacting with Nck)]. Endothelial barrier function Thomas Luft, is moderated by the function of transmembrane receptors and signaling events that act University Hospital Heidelberg, Germany to defend or destabilize it. Here, I highlight recent advances that have provided new Reviewed by: insights into endothelial barrier function and mechanisms involved. Further investigation Luiza Guilherme, of these mechanisms could lead to the discovery of novel therapeutic targets for human University of São Paulo, Brazil diseases associated with endothelial dysfunction. -

Cadherin-Mediated Adhesion Is Essential for Myofibril Continuity Across the Plasma Membrane but Not for Assembly of the Contract
Research Article 1471 Cadherin-mediated adhesion is essential for myofibril continuity across the plasma membrane but not for assembly of the contractile apparatus Yang Luo and Glenn L. Radice* Center for Research on Reproduction and Women’s Health, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA *Author for correspondence (e-mail: [email protected]) Accepted 23 December 2002 Journal of Cell Science 116, 1471-1479 © 2003 The Company of Biologists Ltd doi:10.1242/jcs.00339 Summary The strong coordinated contraction of heart muscle is however, alignment of the myofibrils through regions of dependent on the correct alignment and connection of the cell-cell contact was lost, resulting in their random myofibrils across the plasma membrane. Previous studies orientation. Gap junctions were perturbed in the N- indicate that N-cadherin is involved in cardiac myocyte cadherin-null myocytes. By contrast, focal contacts adhesion and myofibrillogenesis. To investigate whether N- appeared normal in the mutant cells. Furthermore, E- cadherin is specifically required for normal myocyte cadherin restored normal cell morphology and behavior structure and function, we cultured myocytes from wild- to the N-cadherin-deficient myocytes, including proper type, N-cadherin-null and mutant embryos expressing the alignment of the myofibrils. We conclude that a different epithelial cadherin E-cadherin. In contrast to previous adhesive system, most probably integrin, is responsible for studies in chicken using N-cadherin-perturbing antibodies, myofibrillogenesis in the N-cadherin-null myocytes. our in vitro studies with mouse cells demonstrate that N- cadherin is not required for myofibrillogenesis, but is critical for myofibril organization. -

Estudio Del Receptor 2 De La Dopamina En Ovario Humano Y Efecto De Su Modulación Sobre El Síndrome De Hiperestimulación Ovárica”
Facultad de Medicina y Odontología Departamento de Pediatría, Obstetricia y Ginecología. “Estudio del receptor 2 de la dopamina en ovario humano y efecto de su modulación sobre el Síndrome de Hiperestimulación Ovárica” Tesis doctoral presentada por: Francisco Manuel Delgado Rosas Dirigida por: Prof. Antonio Pellicer Martínez Dr. Raúl Gómez Gallego Prof. Francisco Gaytán Luna Tutor: Prof. Carlos Simón Vallés 290 F OBSTETRICIA I GINECOLOGIA II Valencia 2012 D. Antonio Pellicer Martínez, Catedrático del Departamento de Pediatría, Obstetricia y Ginecología de la Facultad de Medicina de la Universidad de Valencia. D. Raúl Gómez Gallego, Doctor en Ciencias Biológicas e Investigador contratado por la Fundación IVI. Valencia D. Francisco Gaytán Luna, Catedrático del Departamento de Biología Celular, Fisiología e Inmunología de la Facultad de Medicina de la Universidad de Córdoba. CERTIFICAN: Que el trabajo titulado: “Estudio del receptor 2 de la dopamina en ovario humano y efecto de su modulación sobre el Síndrome de Hiperestimulación Ovárica” ha sido realizado íntegramente por D. Francisco Manuel Delgado Rosas bajo nuestra supervisión. Dicho trabajo está concluido y reúne todos los requisitos para su presentación y defensa como TESIS DOCTORAL ante un tribunal. Y para que conste así a los efectos oportunos, firmamos la presente certificación en Valencia a 22 de Febrero de 2012. Fdo. Prof. Antonio Pellicer Martínez Fdo. Dr. Raúl Gómez Gallego Fdo. Prof. Francisco Gaytán Luna LISTA DE ABREVIATURAS AII: Angiotensina II ACE: Enzima convertidora de -

Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M
14 The Open Dermatology Journal, 2010, 4, 14-20 Open Access Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M. Brandner1,§, Marek Haftek*,2,§ and Carien M. Niessen3,§ 1Department of Dermatology and Venerology, University Hospital Hamburg-Eppendorf, Hamburg, Germany 2University of Lyon, EA4169 Normal and Pathological Functions of Skin Barrier, E. Herriot Hospital, Lyon, France 3Department of Dermatology, Center for Molecular Medicine, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Germany Abstract: The skin is an indispensable barrier which protects the body from the uncontrolled loss of water and solutes as well as from chemical and physical assaults and the invasion of pathogens. In recent years several studies have suggested an important role of intercellular junctions for the barrier function of the epidermis. In this review we summarize our knowledge of the impact of adherens junctions, (corneo)-desmosomes and tight junctions on barrier function of the skin. Keywords: Cadherins, catenins, claudins, cell polarity, stratum corneum, skin diseases. INTRODUCTION ADHERENS JUNCTIONS The stratifying epidermis of the skin physically separates Adherens junctions are intercellular structures that couple the organism from its environment and serves as its first line intercellular adhesion to the cytoskeleton thereby creating a of structural and functional defense against dehydration, transcellular network that coordinate the behavior of a chemical substances, physical insults and micro-organisms. population of cells. Adherens junctions are dynamic entities The living cell layers of the epidermis are crucial in the and also function as signal platforms that regulate formation and maintenance of the barrier on two different cytoskeletal dynamics and cell polarity. -

Tight Junction-Based Epithelial Microenvironment and Cell Proliferation
Oncogene (2008) 27, 6930–6938 & 2008 Macmillan Publishers Limited All rights reserved 0950-9232/08 $32.00 www.nature.com/onc REVIEW Tight junction-based epithelial microenvironment and cell proliferation S Tsukita1, Y Yamazaki, T Katsuno, A Tamura and S Tsukita2 Laboratory of Biological Science, Graduate School of Frontier Biosciences/Graduate School of Medicine, Osaka University, Suita, Osaka, Japan Belt-like tight junctions (TJs), referred to as zonula bodies of multicellular organisms. The sheet-like divi- occludens, have long been regarded as a specialized sions allow diffusion barrier formation and selective differentiation of epithelial cell membranes. They are permeation of substances and ions (permselectivity), required for cell adhesion and paracellular barrier func- both excluding unnecessary or toxic molecules and tions, and are now thought to be partly involved in fence including necessary components to maintain home- functions and in cell polarization. Recently, the molecular ostasis. The combination of the belt-like adherens bases of TJs have gradually been unveiled. TJs are junctions (AJs) and tight junctions (TJs) have important constructed by TJ strands, whose basic frameworks are functions in the formation of epithelial cell sheets and composed of integral membrane proteins with four also in the formation of paracellular permselective transmembrane domains, designated claudins. The claudin barriers through their functions as septa (Mitic and family is supposedly composed of at least 24 members in Anderson, 1998; Tsukita and Furuse, 1999; Hartsock mice and humans. Other types of integral membrane and Nelson, 2008). Paracellular barrier functions with proteins with four transmembrane domains, namely occlu- permselectivity are unique to the epithelial cell system din and tricellulin, as well as the single transmembrane and regulate the internal homeostasis of ions and solutes proteins, JAMs (junctional adhesion molecules)and CAR in the body. -

Cell Adhesion Molecules in Normal Skin and Melanoma
biomolecules Review Cell Adhesion Molecules in Normal Skin and Melanoma Cian D’Arcy and Christina Kiel * Systems Biology Ireland & UCD Charles Institute of Dermatology, School of Medicine, University College Dublin, D04 V1W8 Dublin, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +353-1-716-6344 Abstract: Cell adhesion molecules (CAMs) of the cadherin, integrin, immunoglobulin, and selectin protein families are indispensable for the formation and maintenance of multicellular tissues, espe- cially epithelia. In the epidermis, they are involved in cell–cell contacts and in cellular interactions with the extracellular matrix (ECM), thereby contributing to the structural integrity and barrier for- mation of the skin. Bulk and single cell RNA sequencing data show that >170 CAMs are expressed in the healthy human skin, with high expression levels in melanocytes, keratinocytes, endothelial, and smooth muscle cells. Alterations in expression levels of CAMs are involved in melanoma propagation, interaction with the microenvironment, and metastasis. Recent mechanistic analyses together with protein and gene expression data provide a better picture of the role of CAMs in the context of skin physiology and melanoma. Here, we review progress in the field and discuss molecular mechanisms in light of gene expression profiles, including recent single cell RNA expression information. We highlight key adhesion molecules in melanoma, which can guide the identification of pathways and Citation: D’Arcy, C.; Kiel, C. Cell strategies for novel anti-melanoma therapies. Adhesion Molecules in Normal Skin and Melanoma. Biomolecules 2021, 11, Keywords: cadherins; GTEx consortium; Human Protein Atlas; integrins; melanocytes; single cell 1213. https://doi.org/10.3390/ RNA sequencing; selectins; tumour microenvironment biom11081213 Academic Editor: Sang-Han Lee 1.