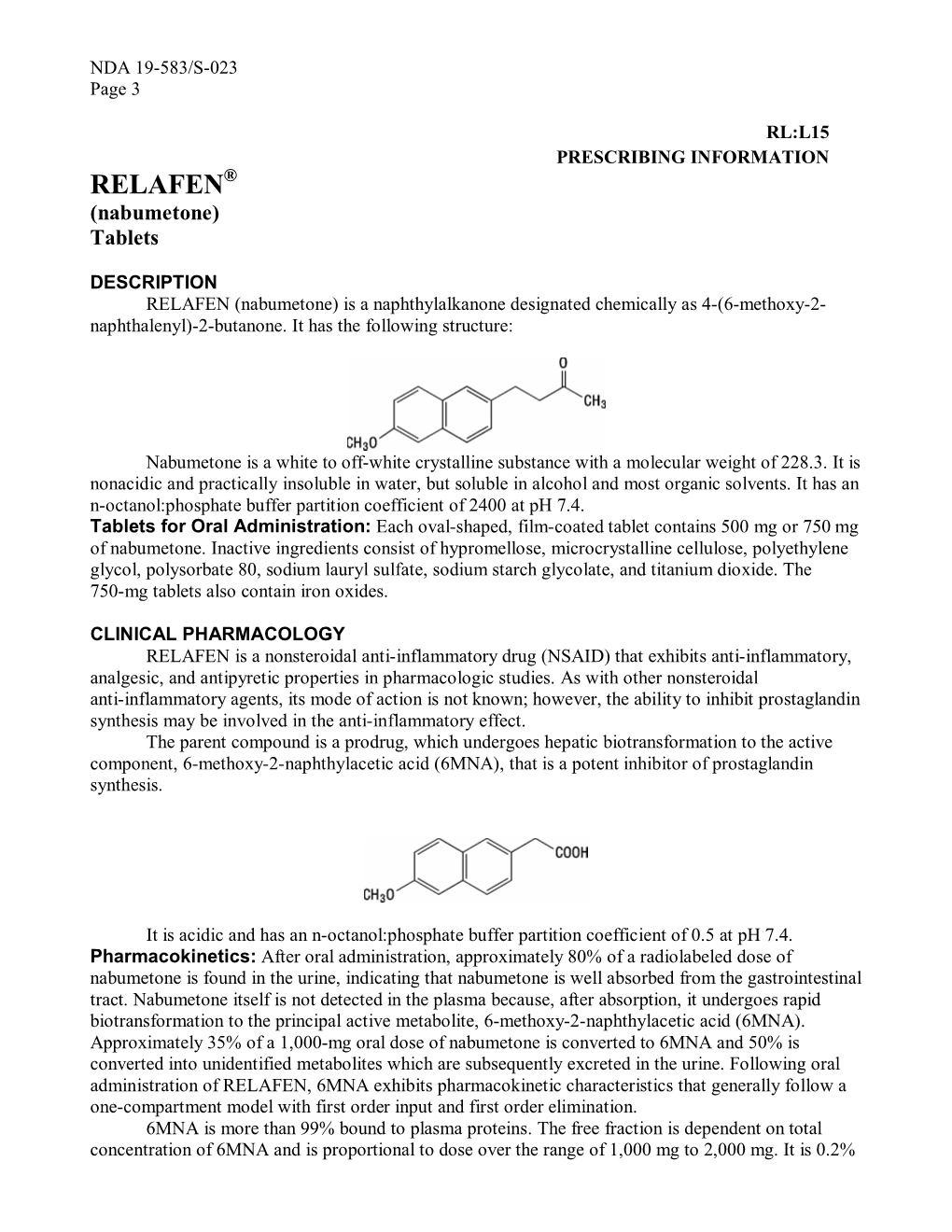
RELAFEN® (Nabumetone) Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nsaids: Dare to Compare 1997
NSAIDs TheRxFiles DARE TO COMPARE Produced by the Community Drug Utilization Program, a Saskatoon District Health/St. Paul's Hospital program July 1997 funded by Saskatchewan Health. For more information check v our website www.sdh.sk.ca/RxFiles or, contact Loren Regier C/O Pharmacy Department, Saskatoon City Hospital, 701 Queen St. Saskatoon, SK S7K 0M7, Ph (306)655-8506, Fax (306)655-8804; Email [email protected] We have come a long way from the days of willow Highlights bark. Today salicylates and non-steroidal anti- • All NSAIDs have similar efficacy and side inflammatory drugs (NSAIDs) comprise one of the effect profiles largest and most commonly prescribed groups of • In low risk patients, Ibuprofen and naproxen drugs worldwide.1 In Saskatchewan, over 20 may be first choice agents because they are different agents are available, accounting for more effective, well tolerated and inexpensive than 300,000 prescriptions and over $7 million in • Acetaminophen is the recommended first line sales each year (Saskatchewan Health-Drug Plan agent for osteoarthritis data 1996). Despite the wide selection, NSAIDs • are more alike than different. Although they do Misoprostol is the only approved agent for differ in chemical structure, pharmacokinetics, and prophylaxis of NSAID-induced ulcers and is to some degree pharmacodynamics, they share recommended in high risk patients if NSAIDS similar mechanisms of action, efficacy, and adverse cannot be avoided. effects. week or more to become established. For this EFFICACY reason, an adequate trial of 1-2 weeks should be NSAIDs work by inhibiting cyclooxygenase (COX) allowed before increasing the dose or changing to and subsequent prostaglandin synthesis as well as another NSAID. -

(Ketorolac Tromethamine Tablets) Rx Only WARNING TORADOL
TORADOL ORAL (ketorolac tromethamine tablets) Rx only WARNING TORADOLORAL (ketorolac tromethamine), a nonsteroidal anti-inflammatory drug (NSAID), is indicated for the short-term (up to 5 days in adults), management of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following IV or IM dosing of ketorolac tromethamine, if necessary. The total combined duration of use of TORADOLORAL and ketorolac tromethamine should not exceed 5 days. TORADOLORAL is not indicated for use in pediatric patients and it is NOT indicated for minor or chronic painful conditions. Increasing the dose of TORADOLORAL beyond a daily maximum of 40 mg in adults will not provide better efficacy but will increase the risk of developing serious adverse events. GASTROINTESTINAL RISK Ketorolac tromethamine, including TORADOL can cause peptic ulcers, gastrointestinal bleeding and/or perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Therefore, TORADOL is CONTRAINDICATED in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS). CARDIOVASCULAR RISK NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS and CLINICAL STUDIES). TORADOL is CONTRAINDICATED for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). -

Non Steroidal Anti-Inflammatory Drugs
Non Steroidal Anti‐inflammatory Drugs (NSAIDs) 4 signs of inflammation • Redness ‐ due to local vessel dilatation • Heat ‐ due to local vessel dilatation • Swelling – due to influx of plasma proteins and phagocytic cells into the tissue spaces • Pain – due to local release of enzymes and increased tissue pressure NSAIDs • Cause relief of pain ‐. analgesic • Suppress the signs and symptoms of inflammation. • Exert antipyretic action. • Useful in pain related to inflammation. Esp for superficial/integumental pain . Classification of NSAIDs • Salicylates: aspirin, Sodium salicylate & diflunisal. • Propionic acid derivatives: ibuprofen, ketoprofen, naproxen. • Aryl acetic acid derivatives: diclofenac, ketorolac • Indole derivatives: indomethacin, sulindac • Alkanones: Nabumetone. • Oxicams: piroxicam, tenoxicam Classification of NSAIDs ….. • Anthranilic acid derivatives (fenamates): mefenamic acid and flufenamic acid. • Pyrazolone derivatives: phenylbutazone, oxyphenbutazone, azapropazone (apazone) & dipyrone (novalgine). • Aniline derivatives (analgesic only): paracetamol. Clinical Classif. • Non selective Irreversible COX inhibitors • Non slective Reversible COX inhibitors • Preferential COX 2 inhibitors • 10‐20 fold cox 2 selective • meloxicam, etodolac, nabumetone • Selective COX 2 inhibitors • > 50 fold COX ‐2 selective • Celecoxib, Etoricoxib, Rofecoxib, Valdecoxib • COX 3 Inhibitor? PCM Cyclooxygenase‐1 (COX‐1): -constitutively expressed in wide variety of cells all over the body. -"housekeeping enzyme" -ex. gastric cytoprotection, hemostasis Cyclooxygenase‐2 (COX‐2): -inducible enzyme -dramatically up-regulated during inflammation (10-18X) -constitutive : maintains renal blood flow and renal electrolyte homeostasis Salicylates Acetyl salicylic acid (aspirin). Kinetics: • Well absorbed from the stomach, more from upper small intestine. • Distributed all over the body, 50‐80% bound to plasma protein (albumin). • Metabolized to acetic acid and salicylates (active metabolite). • Salicylate is conjugated with glucuronic acid and glycine. • Excreted by the kidney. -

Parkinson's Disease Research
NGRC EEQ ENVIRONMENTAL EXPOSURE QUESTIONNAIRE page 1of 2 Tobacco Have you smoked at least 100 cigarettes (about 5 packs) in your entire life time? Yes No (If no, go to NSAIDs section) During the time that you smoked, how much did you smoke on average, and for how many years? Check all that apply. Less than ½ pack per day, for _____ years (specify number of years) Equal to or more than ½ pack but less than 1 pack per day, for _____ years (specify number of years) Equal to or more than 1 pack but less than 2 packs per day, for _____ years (specify number of years) Equal to or more than 2 packs per day, for _____ years (specify number of years) At what age did you begin smoking? _________ Are you still smoking? Yes No If not, at what age did you stop? _______________ NSAIDs NSAIDs are non-steroidal anti-inflammatory drugs, like Ibuprofen, Motrin IB, Advil and Aleve, which are commonly used for pain. Aspirin and acetaminophens like Tylenol are not NSAIDs. How often do you (or did you) take over the counter NSAIDs, and for how many years? Check all that apply. Never Less than once a week, for _____ years (specify number of years) About one to four times a week, for _____ years (specify number of years) About 5 to 10 times a week, for _____ years (specify number of years) More than 10 times a week, for _____ years (specify number of years) How often do you (or did you) take prescription NSAIDs, and for how many years? Check all that apply. -

Inflammatory Drugs (Nsaids) for People with Or at Risk of COVID-19
Evidence review Acute use of non-steroidal anti- inflammatory drugs (NSAIDs) for people with or at risk of COVID-19 Publication date: April 2020 This evidence review sets out the best available evidence on acute use of non- steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. It should be read in conjunction with the evidence summary, which gives the key messages. Evidence review commissioned by NHS England Disclaimer The content of this evidence review was up-to-date on 24 March 2020. See summaries of product characteristics (SPCs), British national formulary (BNF) or the MHRA or NICE websites for up-to-date information. For details on the date the searches for evidence were conducted see the search strategy. Copyright © NICE 2020. All rights reserved. Subject to Notice of rights. ISBN: 978-1-4731-3763-9 Contents Contents ...................................................................................................... 1 Background ................................................................................................. 2 Intervention .................................................................................................. 2 Clinical problem ........................................................................................... 3 Objective ...................................................................................................... 3 Methodology ................................................................................................ 4 Summary of included studies -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Non-Selective Oral Nonsteroidal Anti-Inflammatory Drugs AHFS Classification: 28:08.04.92 Other Nonsteroidal Anti-inflammatory Agents Diclofenac Etodolac Fenoprofen Flurbiprofen Ibuprofen Indomethacin Ketoprofen Ketorolac Mefenamic Acid Meloxicam Nabumetone Naproxen Oxaprozin Piroxicam Sulindac Tolmetin Final Report April 2018 Review prepared by: Elena Martinez Alonso, B.Pharm., Medical Writer Vicki Frydrych, B.Pharm., Pharm.D., Clinical Pharmacist Valerie Gonzales, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor, Clinical Pharmacist Michelle Fiander, MA, MLIS, Research Assistant Professor, Evidence Synthesis Librarian Joanne LaFleur, PharmD, MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved 1 Contents Executive Summary ...................................................................................................................................... 3 Introduction ................................................................................................................................................... 6 Table 1. FDA-Approved Oral Non-selective NSAIDs......................................................................... 7 Table 2. FDA-Approved Indication for Oral Non-selective NSAIDs ............................................... -

Dolobid® (Diflunisal)
9676203 TABLETS DOLOBID® (DIFLUNISAL) Cardiovascular Risk • NSAIDS may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (See WARNINGS.) • DOLOBID is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). Gastrointestinal Risk • NSAIDS cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS.) DESCRIPTION Diflunisal is 2', 4'-difluoro-4-hydroxy-3-biphenylcarboxylic acid. Its empirical formula is C13H8F2O3 and its structural formula is: Diflunisal has a molecular weight of 250.20. It is a stable, white, crystalline compound with a melting point of 211-213°C. It is practically insoluble in water at neutral or acidic pH. Because it is an organic acid, it dissolves readily in dilute alkali to give a moderately stable solution at room temperature. It is soluble in most organic solvents including ethanol, methanol, and acetone. DOLOBID* (Diflunisal) is available in 250 and 500 mg tablets for oral administration. Tablets DOLOBID contain the following inactive ingredients: cellulose, FD&C Yellow 6, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, starch, talc, and titanium dioxide. CLINICAL PHARMACOLOGY Action DOLOBID is a non-steroidal drug with analgesic, anti-inflammatory and antipyretic properties. -

Indocin® (Indomethacin) Oral Suspension
INDOCIN® (INDOMETHACIN) ORAL SUSPENSION Cardiovascular Risk • NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at a greater risk. (See WARNINGS.) • INDOCIN is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). Gastrointestinal Risk • NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS.) DESCRIPTION Suspension INDOCIN1 for oral use contains 25 mg of indomethacin per 5 mL, alcohol 1%, and sorbic acid 0.1% added as a preservative and the following inactive ingredients: antifoam AF emulsion, flavors, purified water, sodium hydroxide or hydrochloric acid to adjust pH, sorbitol solution, and tragacanth. Indomethacin is a non-steroidal anti-inflammatory indole derivative designated chemically as 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid. 1 Indomethacin is practically insoluble in water and sparingly soluble in alcohol. It has a pKa of 4.5 and is stable in neutral or slightly acidic media and decomposes in strong alkali. The suspension has a pH of 4.0-5.0. The structural formula is: 1 Registered trademark of MERCK & CO., Inc., Whitehouse Station, NJ U.S.A. and licensed to Iroko Pharmaceuticals, LLC, Philadelphia, PA, U.S.A. -

Pulmonary Fibrosis Associated with Nabumetone A
Postgrad Med J (1991) 67, 1021 - 1022 © The Fellowship of Postgraduate Medicine, 1991 Postgrad Med J: first published as 10.1136/pgmj.67.793.1021 on 1 November 1991. Downloaded from Pulmonary fibrosis associated with nabumetone A. Moricpl*, A. Atherton', F. Gleeson2 and S. Stewart3 'Chest Medical Unit and 3Department ofHistopathology, Papworth Hospital, Papworth Everard, Cambs and 2Department ofRadiology, Addenbrooke's Hospital, Cambridge, UK Summary: A patient is described who developed a rapid onset of pulmonary fibrosis following treatment with a new non-steroidal anti-inflammatory drug, nabumetone. Resolution of symptoms, physical signs and radiographic changes followed drug withdrawal and steroid therapy. Introduction Pulmonary infiltration and fibrosis have been des- cribed with a number of non-steroidal anti-infla- matory agents.' We report a case where an unusual radiographic appearance ofpulmonary infiltration was associated with drug therapy. Case report by copyright. A 68 year old woman with osteoarthritis and osteoporosis was treated with paracetamol and dextropropoxyphene (Co-proxamol), ibuprofen 1,200 mg/day and calcium lactate gluconate (San- docal) for 4 years. Tiaprofenic acid 600 mg/day was added but gastric disturbance lead to a change in her analgesia to nabumetone 1500 mg/day. Two Figure 1 Representative 4 mm transaxial computed weeks later she developed shortness ofbreath and a tomography scan through the lung bases. dry cough. Breathlessness increased for 6 weeks until she was short of breath on minimal exertion. demonstrated bizarre pulmonary shadowing pre- She was a non-smoker without recognized risk dominantly in the lower zones, without subpleural factors for fibrotic lung disease. bias or evidence ofbronchiectasis and with sparing http://pmj.bmj.com/ On examination there was no cyanosis or clubb- ofthe lung apices. -

Ketorolac Tromethamine Injection Usp
PRODUCT MONOGRAPH PrKETOROLAC TROMETHAMINE INJECTION USP (Ketorolac Tromethamine) Sterile Solution 30 mg/mL Non-Steroidal Anti-Inflammatory Drug (NSAID) Pfizer Canada Inc. Date of Revision: 17300 Trans-Canada Highway December 1, 2017 Kirkland, Québec H9J 2M5 Submission Control No: 209949 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................5 ADVERSE REACTIONS ..................................................................................................14 DRUG INTERACTIONS ..................................................................................................17 DOSAGE AND ADMINISTRATION ..............................................................................19 OVERDOSAGE ................................................................................................................20 ACTION AND CLINICAL PHARMACOLOGY ............................................................21 STORAGE AND STABILITY ..........................................................................................24 DOSAGE FORMS, COMPOSITION AND PACKAGING -

Oral Nonsteroidal Anti-Inflammatory Drugs (Nsaids)
Therapeutic Class Overview Oral nonsteroidal anti-inflammatory drugs (NSAIDs) INTRODUCTION Nonsteroidal anti-inflammatory drugs (NSAIDs) are a large class of medications with analgesic, anti-inflammatory, and anti-pyretic properties used for a wide variety of conditions including pain, rheumatoid arthritis (RA), osteoarthritis (OA), primary dysmenorrhea, ankylosing spondylitis (AS), juvenile idiopathic arthritis (JIA), acute migraine, and acute gout (Conaghan 2012). ○ RA is an autoimmune inflammatory arthritis that can be treated with conventional or biologic disease-modifying antirheumatic drugs (DMARDs) such as Trexall (methotrexate) or Humira (adalimumab), systemic or intraarticular (IA) corticosteroids, and/or oral or topical analgesics including NSAIDs (Singh et al 2015). ○ OA is the most common form of arthritis, and is a degenerative inflammatory disease that can be treated with oral or topical analgesics including NSAIDs, IA corticosteroid or hyaluronate injections, Cymbalta (duloxetine), and physical therapy (Hochberg et al 2012, Loeser 2018). ○ Primary dysmenorrhea is menstrual pain in the absence of other pelvic pathology, and represents one of the most common causes of pelvic pain. It can be treated with oral NSAIDs, hormonal contraceptives, topical heat, and exercise (Osayande et al 2013). ○ AS is a chronic inflammatory arthritis characterized by sacro-iliac joint involvement that can be treated with oral or topical NSAIDs, tumor necrosis factor inhibitors, slow acting antirheumatic drugs including Plaquenil (hydroxychloroquine), locally administered parenteral glucocorticoids, physical therapy, or surgery (Ward et al 2016). ○ JIA is a chronic idiopathic inflammatory disorder that affects pediatric patients. JIA encompasses multiple forms of arthritis in childhood, including what was previously described as juvenile rheumatoid arthritis before being supplanted by the newer term. -

8296901/0315 MEDICATION GUIDE for NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) (See the End of This Medication Guide for a List of Prescription NSAID Medicines.)
8296901/0315 MEDICATION GUIDE FOR NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) (See the end of this Medication Guide for a list of prescription NSAID medicines.) Rx only What is the most important information I should know about medicines called Non-Steroidal Anti- Inflammatory Drugs (NSAIDs)? NSAID medicines may increase the chance of a heart attack or stroke that can lead to death. This chance increases: o with longer use of NSAID medicines o in people who have heart disease NSAID medicines should never be used right before or after a heart surgery called a “coronary artery bypass graft (CABG).” NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding: o can happen without warning symptoms o may cause death The chance of a person getting an ulcer or bleeding increases with: o taking medicines called “corticosteroids” and “anticoagulants” o longer use o smoking o drinking alcohol o older age o having poor health NSAID medicines should only be used: o exactly as prescribed o at the lowest dose possible for your treatment o for the shortest time needed What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)? NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as: o different types of arthritis o menstrual cramps and other types of short-term pain Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)? Do not take an NSAID medicine: o if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine o for pain right before or after heart bypass surgery Tell your healthcare provider: o about all of your medical conditions.